Breast carcinoma is the most common malignancy globally and one of the leading causes of cancer-related deaths among women.[1] The highest incidence of breast carcinoma among Asian nations is found in Pakistan. One in nine women in Pakistan is expected to get a breast cancer diagnosis at some point in their lives.[2] Approximately 3% of cancer diagnoses and fatalities globally are related to non-Hodgins’ lymphoma (NHL), the most prevalent hematological cancer. NHL has the sixth-highest cancer-related death rate and is the seventh most common malignancy in the US. Since 1975, the incidence of NHL has increased by 168% (despite improvements in survival of 158%), making up 4% of cancer diagnoses in the US.[3] It is uncommon for breast cancer and NHL to present simultaneously.[4] An increase in the rate of simultaneous malignancy identification has resulted from the adoption of more sensitive staging imaging techniques.[5] In this instance, we describe a woman who, during the staging process for possible breast cancer, was found to have axillary nodal diffuse large B cell lymphoma (DLBCL) in a cancer hospital.
A 54-year-old post-menopausal female presented in March 2023 with a complaint of an isolated right breast lump for 5 months. She had no B symptoms on presentation. She had no significant comorbidities or any significant medication history. Her family history of cancer was negative. Her general physical and systemic examination was unremarkable, apart from a 2.5 cm mass in the right breast upper outer quadrant with an enlarged right axillary lymph node.
Ultrasound right breast showed a 25–27 mm mass at the 10 O’clock position and lymphadenopathy in the right axilla. A core biopsy from the right breast tissue revealed invasive ductal carcinoma of the breast (Grade II as shown in Figure 2). In contrast, the right axillary lymph node core biopsy showed DLBCL. On immunohistochemistry, CD20 was positive, whereas CD10 and CD5 were negative. Ki67 was 50% (Figure 1). Baseline work-up including positron emission tomography scan, estrogen receptor (ER), progesterone receptor (PR), and Her-2 neu on breast biopsy specimen were ordered. ER was reported to be 95%, PR 50%, and Her-2 neu status was positive on breast biopsy. Positron emission tomography showed fluorodeoxyglucose avid right breast mass and axillary lymph node as can be seen in Figure 3. Mildly avid subcentimetric, mediastinal, and hilar lymphadenopathy was also seen. Her case was discussed in a multidisciplinary tumor board meeting. Breast cancer was staged as cT2N0/Stage IIA and DLBCL as Stage IE. It was planned to start her with rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisolone (R-CHOP) for three cycles followed by interim positron emission tomography; if the patient has a complete metabolic response (CMR), then the plan was to give one more cycle of R-CHOP followed by breast-conserving surgery. Adjuvant paclitaxel, Her-2 targeted therapy, and radiation were to be given after surgery. Interim positron emission tomography scan after three cycles of R-CHOP showed a complete metabolic response (Deauville score 2 as visualized in Figure 4). She was given one more cycle of R-CHOP. Then, she had right breast-conserving surgery with axillary lymph node dissection in August 2023. Histopathology was reported as residual invasive breast carcinoma, no special type (ductal), grade-3, 23 mm with associated ductal carcinoma in situ, solid pattern, intermediate nuclear grade. Skin and all resection margins were free of tumors. Lymphovascular invasion was seen and two of two lymph nodes were positive for metastatic carcinoma on the frozen section, and one of 16 lymph nodes was positive for metastatic carcinoma with extranodal extension (ypT2ypN1a). She was recommended weekly paclitaxel for 12 cycles and trastuzumab and pertuzumab for 1 year. She is currently having her adjuvant trastuzumab/pertuzumab and paclitaxel, after which she will be planned for right breast and supraclavicular radiation with 40.5 Gy/15 fractions. Endocrine therapy will be started once chemotherapy is completed. She will be given artificial intelligence for an extended duration in view of the reported improvement in disease-free survival. She will be given endocrine therapy for extended duration in view of the reported improvement in disease free survival.
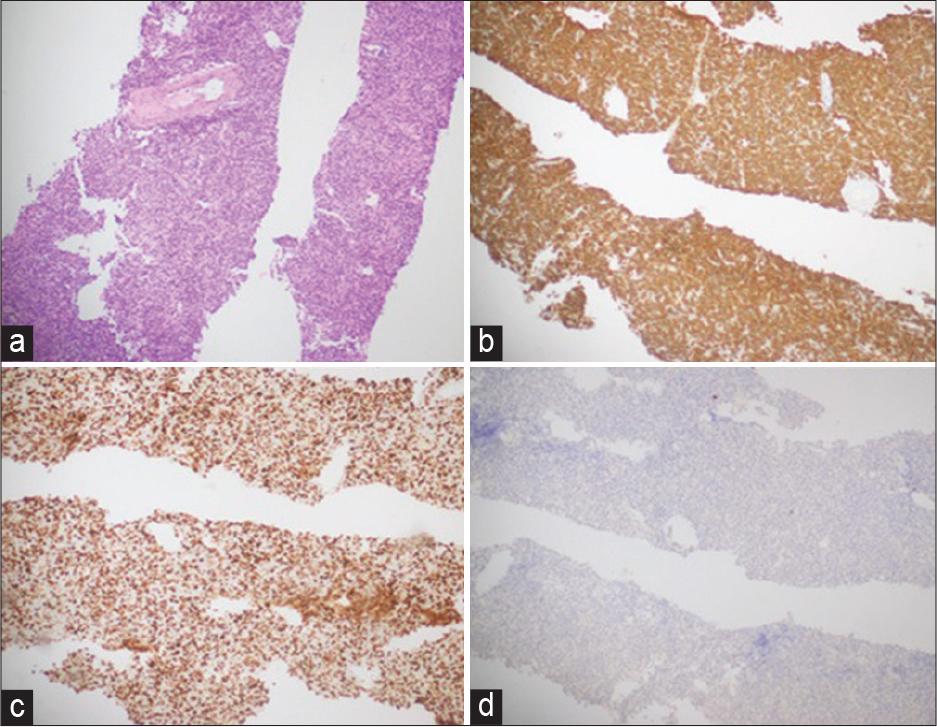
(a) Right axillary lymph node trucut biopsy; diffuse large B-cell lymphoma. (b) CD 20: Positive in tumor cells. (c) Ki67; 50% proliferation index in tumor cells. (d) Cytokeratin; negative in tumor cells
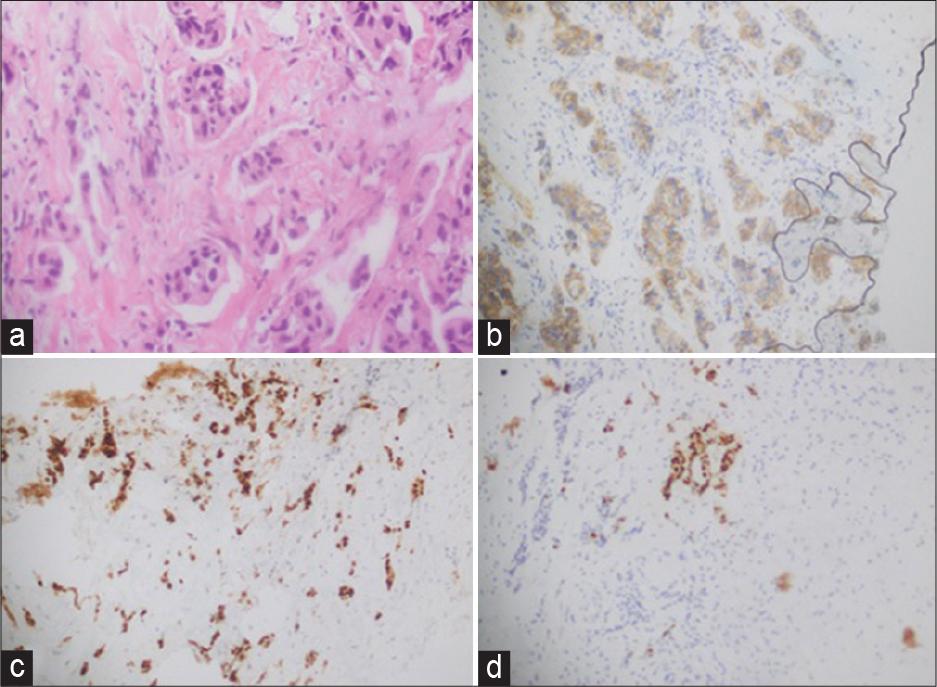
(a) Right breast trucut biopsy: Invasive breast carcinoma, no special type (ductal), grade II. (b) Her 2: Equivocal: Score 2+ (uniform, intense complete membranous staining in: 5–10% cells). (c) Estrogen receptor; positive, strong staining in 95% of tumor cells. (d) progesterone receptor; positive, strong staining in 50% of tumor cells
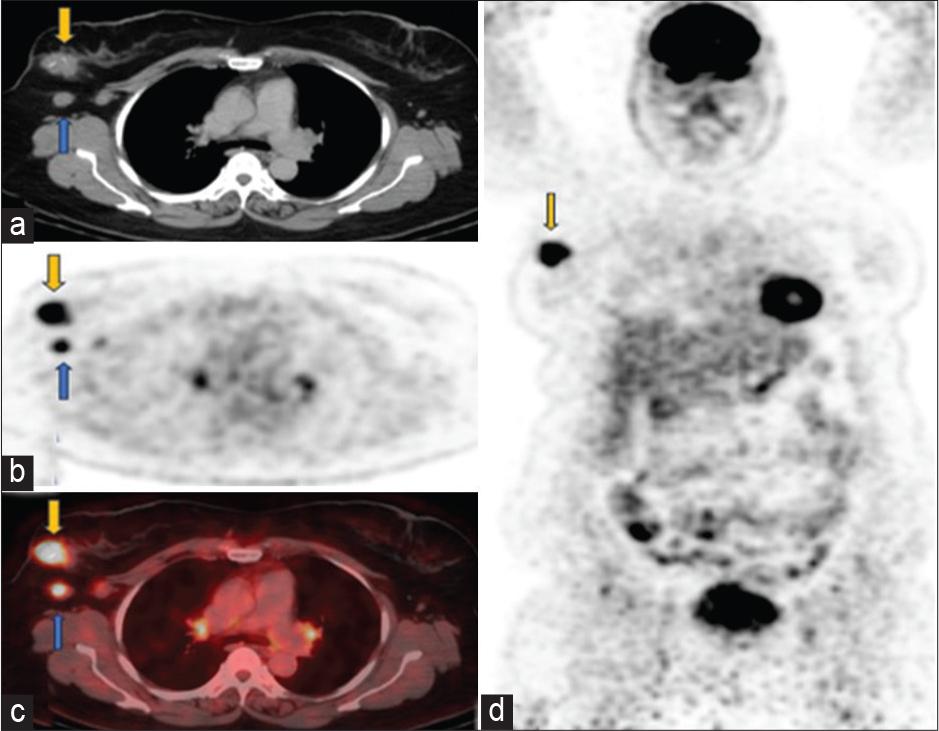
Baseline scan April 2023 (a) axial computed tomography (CT), (b) positron emission tomography only, (c) fused positron emission tomography-CT images through the chest showed fludeoxyglucose (FDG) avid soft tissue mass in the upper outer quadrant of right breast (yellow arrow) has SUV 7.4. FDG avid right axillary lymph nodes (SUV 3.7) are marked with blue arrows, consistent with DLBCL. Small volume mildly avid mediastinal and hilar lymph nodes are likely reactive. (d) Coronal positron emission tomography-only images show hypermetabolic right breast biopsy-proven ductal carcinoma in situ
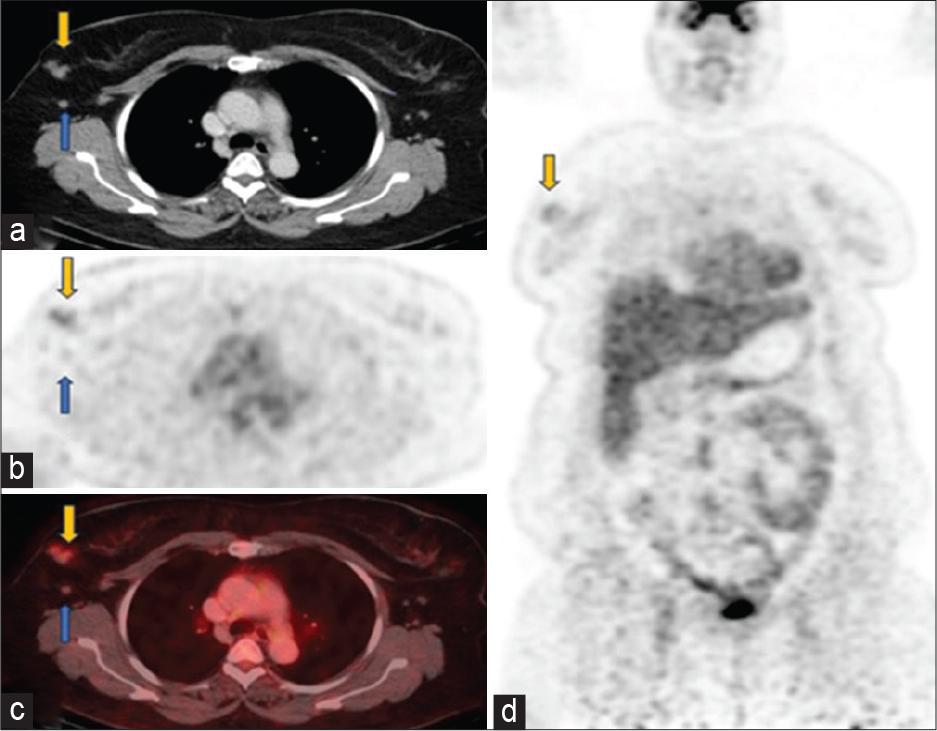
Follow-up scan June 2023 (a) axial computed tomography (CT), (b) positron emission tomography only, (c) fused positron emission tomography-CT images through the chest showed significant interval decrease in size and metabolic activity of right breast mgass (yellow arrows) with faint uptake (SUV 2.6). Right axillary lymphadenopathy has also significantly reduced leaving behind residual non-avid (SUV 1.2) subcentimeter nodes (blue arrows). (d) Coronal positron emission tomography-only images showed a faintly avid residual tumor in the right breast
Lymphoma can develop after breast cancer treatment with chemotherapy and radiation. Nonetheless, it is uncommon for breast cancer and lymphoma to coexist simultaneously, which can make diagnosis and treatment extremely difficult.[4] Case studies and series exist on patients with primary breast cancer who also have concurrent lymphoproliferative disorders such as DLBCL, mantle cell lymphoma, small cell lymphoma, and follicular lymphoma; nevertheless, it is uncommon for invasive breast carcinoma and DLBCL to appear at the same time.[5–8]
Particular diagnostic criteria for multiple primary malignancies (MPM) were proposed by Warren and Gates in 1962. First, there needs to be definitive histologic evidence of malignancy in both tumors; second, there needs to be a histological difference between the two tumors; and third, the second tumor cannot be a metastasis of the first tumor. An MPM is defined as the presence of two or more primary malignant tumors in a single patient. The prevalence of MPM has varied from 0.52% to 11.2% in various settings.[9]
Regarding previously reported similar cases, it is unclear if lymphoma and breast cancer are directly related. It is hypothesized that several carcinomas could develop in succession because lymphomas can impair immune function and prevent T cell-mediated antineoplastic pathways[8].
Physicians treating patients with atypical presentations, like early-stage breast carcinoma with evidence of distant lymphadenopathy, may consider synchronous cancer instead of metastatic illness. Still, the diagnosis of synchronous pathology could be missed if the patient only has lymphadenopathy in the axillary or regional lymph nodes or if the patient has locally progressed breast carcinoma. Physicians should consider doing an immunohistochemistry panel (CD20, CD3, CD10, BCL6, CD21, or CD23) with or without flow cytometry when there is a suspicion of lymphoma.[8]
The exact mechanism behind the disease’s simultaneous manifestation is unknown. A hematological malignancy that affects the body’s lymphatic system is called lymphoma. Malignant and functionally abnormal lymphocytes result in an immunosuppressed state. This is linked to a higher chance of getting breast cancer and other primary malignancies, such as colorectal cancer.[8] The diagnosis of second occult malignancies has grown due to the increasing use of positron emission tomography scans for cancer staging. According to a recent prospective research, approximately 2.9% of patients with NHL who were staged by positron emission tomography had an undetected non-lymphoma malignancy.[10]
There are recognized protocols for the work-up, staging, and therapy of individual malignancies; however, choosing the optimal course of action when many cancers are present may be difficult. Prioritizing the most critical issue first to treat each tumor as best as possible occurs when competing demands for urgency arise. The National Comprehensive Cancer Network (NCCN) clinical practice guidelines state that the standard of care for the first-line treatment of early-stage, non-bulky (<7.5 cm) DLBCL is systemic chemotherapy with R-CHOP for three cycles, followed by interim positron emission tomography.[11] If the patient achieves cMR, one more cycle of RCHOP and surveillance should be performed.[11]
Neoadjuvant chemotherapy combined with anti-Her 2 targeted therapy is the treatment for breast cancer in patients with a clinically T2 tumor that is also Her 2 neu positive. We modified the systemic chemotherapy for our patient so that, since two of the drugs (doxorubicin and cyclophosphamide) were also effective against invasive ductal carcinoma, the treatment for DLBCL also served as neoadjuvant therapy for the malignancy switch malignancy to breast cancer. Her 2 targeted treatments were planned to be incorporated in an adjuvant setting for 1 year. In our patient, as it was node-positive disease on surgical pathology specimen, Taxol chemotherapy was also added to targeted treatment.
Adjuvant endocrine therapy utilizing aromatase inhibitors such as anastrozole, exemestane, or letrozole or selective ER modulators (SERM) such as tamoxifen is recommended for all patients with hormone receptor-positive breast cancer. According to data, these medicines reduce the chance of recurrence by 50% and mortality by roughly 30%.[8] Aromatase inhibitors are recommended for at least 5 years in postmenopausal women and are preferable over SERM.[8]
The identification of occult concurrent cancers is a real possibility when sensitive staging and diagnostic investigations are used appropriately. Histopathological confirmation of regional nodes and even distant metastasis should be attempted wherever possible for the appropriate management of patients. To optimize the likelihood of curing these malignancies, the most effective treatment strategy must be carefully reviewed, evaluated, and discussed in a multidisciplinary context.[5]
Complete baseline work-up according to standard protocols/guidelines should be done in each malignancy. Biopsy of metastatic sites should be done wherever possible. All histopathologies should be reviewed thoroughly before treatment initiation as it may significantly alter patient management. Reporting such rare cases is important as it might help in the management of such unusual presentations.