Figure 1
The biological parameter changes in trials proving the beneficial effect of stem-cell therapy in myocardial infarction
| TRIAL | CELL TYPE | BIOLOGICAL PARAMETER | |
|---|---|---|---|
| Fernandez-Aviles et al., 2018 19 | Allogenic CSC | CRP | Similar baseline values between groups. |
| NT-proBNP | Similar baseline values. | ||
| CK and TnT | Similar decreasing trend. | ||
| Makkar et al., 2020 20 | Allogenic CDC | NT-proBNP | Greater decreasing level at 6 months in study group (p=0.02). |
| Peregud-Pogorzelska et al., 2020 16 | Autologous BM-MNC | CRP, CK-MB, TnT, BNP | Similar baseline levels between the entire study group and control group but lower CK-MB, TnT, and BNP initial values in responders, with faster decrease in BNP levels in the entire study group and TnT levels in the responders. |
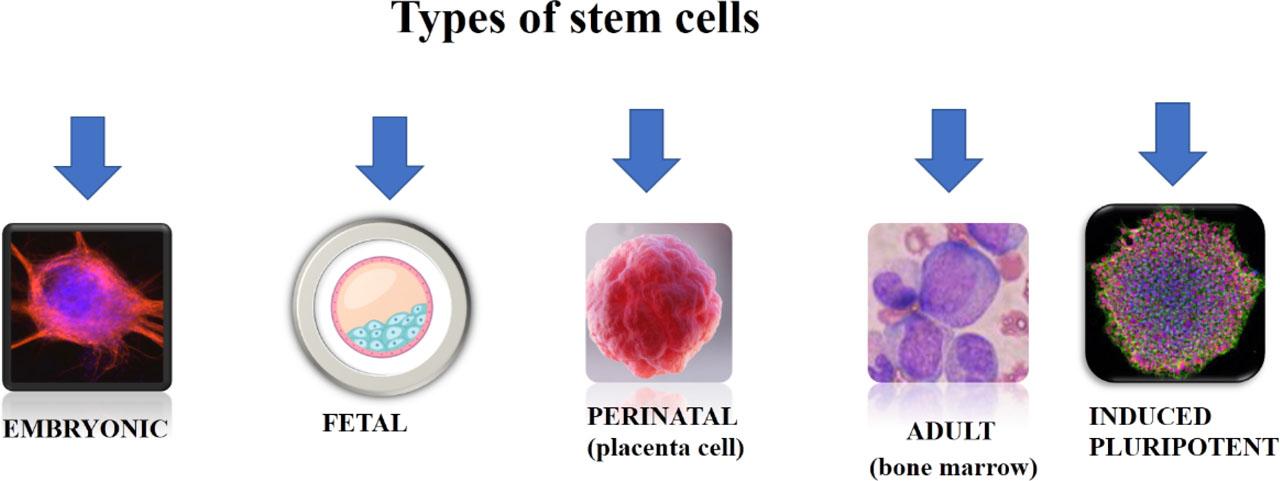
Types of stem cells and their advantages and disadvantages
| CELL TYPE | ORIGIN | ADVANTAGES | DISADVANTAGES |
|---|---|---|---|
| EMBRYONIC | Zygote. | Higher differentiation potency. | Subject to ethical issues due to methods of obtaining them. |
| FETAL | Blastocyst. | High differentiation potency. | Subject to ethical issues due to methods of obtaining them. |
| PERINATAL | Umbilical cord. | Multipotent. | Differentiation potency is lower than that of fetal cells. |
| ADULT | Bone marrow. | Oligopotent. | Differentiation potency is the lowest. |
| INDUCED PLURIPOTENT | Adult somatic cells genetically modified to resemble embryonic stem cells. | Obtained from adult and perinatal cells (not an ethical issue). | Immunogenic effect. |
Types of stem cells and their effect in reviewed studies
| CELL TYPE [ref] | END POINTS | NO. OF PATIENTS ENROLLED | TIME OF INTERVENTION | NO. OF CELLS | ASSESSMENT METHOD FOR EFFICACY | FINDINGS |
|---|---|---|---|---|---|---|
| Autologous BM-MNC [16] | Safety Efficacy | 15 in study group, | 24 hours after STEMI. | 8.37 × 106 | LVEDV, LVESV, LVEF by echocardiography (biplane Simpson method – apical two- and four-chamber). | Achieved safety. |
| Autologous BM-MSC [14] | Safety Efficacy | 14 in study group, | 1 month after STEMI. | 7.2 ± 0.9 × 107 | LVEF by SPECT and echocardiography. | Achieved safety. |
| Autologous BM-MNC [18] | Efficacy | 51 in early study group | Early: 5–7 days after STEMI | 5 × 107 – 5 × 108 | GLS and GCS by cardiac magnetic resonance. | None of time related treatment proved an improvement in cardiac parameters. |
| Autologous BM-MNC [17] | Efficacy | 66 in study group, | 6–9 days after STEMI. | 100 × 106 | LVEF, LVEDV, LVESV, infarct size by cardiac magnetic resonance. | Study and control group had similar results at baseline, at 6-month follow-up and between times. |
| Autologous BM-MSC [10] | Safety Efficacy | 21 in study group, | 14.07 +/−9.53 days after STEMI. | 3.31 ± 1.7 × 106 | LVEF, LVEDV, LVESV by echocardiography (Simpson method) at 12 months and myocardial perfusion and metabolic activity by SPECT at 6-month follow-up. | Achieved safety. |
| Allogenic CSC [19] | Safety Efficacy | 33 in study group | Days 5–7 after STEMI. | 35 × 106 | Infarct size, LVEF, LVEDV, LVESV and wall motion score by cardiac magnetic resonance. | Safety was the primary endpoint, and it didn’t reveal any major cardiac adverse events or deaths at 6- and 12-month follow-up. No significant differences in assessed parameters between the groups at baseline and follow-up times. |
| Allogenic CDC [20] | Safety Efficacy | 90 in study group | 4 weeks to 12 months after STEMI. | 2.5 × 106 | Infarct size, LVEDV, LVESV, LVEF by cardiac magnetic resonance. | Safety endpoint was achieved. |