The taxonomic status of the genus Phasmarhabditis, Andrássy, 1976, has been controversial since its establishment. Sudhaus (1976) referred to it as the Papillosa group within the subgenus Pellioditis in the genus Rhabditis (Sudhaus and Fitch, 2001), and Sudhaus (2011) considered it to be synonymous with the genus Pellioditis Dougherty, 1953, with P. pellio Schneider, 1866 as the type species. The morphology of the two genera (Phasmarhabditis and Pellioditis) is known to be very uniform, with no clear distinguishing characteristics between them to warrant separate generic status (Huang et al., 2015; Nermuť et al., 2016a). In the recent update of the catalog of Rhabditidae by Sudhaus (2023), Phasmarhabditis was listed as a synonym of Pellioditis. This has been supported by the fact that the sequence data for the type species P. pellio belongs to the same clade as Phasmarhabditis (Sudhaus, 2023). In this study, we equally agreed with Sudhaus (2023) and treated Phasmarhabditis as a synonym of Pellioditis.
Except for Pellioditis (=Phasmarhabditis) huizhouensis, Huang et al., 2015, which was recovered and described from rotting leaves, members of the genus have generally been described as terrestrial facultative parasites of gastropods, though P. hermaphrodita (Schneider, 1859; Andrássy 1983) has been shown to reproduce in earthworms (Andrássy, 1983; Rae et al., 2006, 2009; Huang et al., 2015; Tandingan De Ley et al., 2016). Until the recent application of molecular DNA barcoding, species within the group have mostly been diagnosed using morphological characters including female tail shape and length, bursal papillae, spicule length and shape, and the presence and absence of males and morphometrics of dauer juveniles (Nermuť et al., 2016a; Ivanova and Spiridonov, 2023). During a nematological survey conducted in 2024, a cryptic population of Pellioditis with very close morphological resemblance to the recently described P. zhejiangensis (Zhang and Liu, 2020) was recovered from dark, brown-colored cadavers of Protaetia brevitarsis seulensis larvae. A scrutiny of morphological features and molecular DNA barcodes revealed significant differences from P. zhejiangensis. This species, herein designated as Pellioditis koreana n. sp., is described considering both morphological and molecular phylogenetic comparisons. The new species represents the first report of an entomo-parasitic relationship within this predominantly gastropod-parasitic genus.
The nematode population was extracted from dark brown-colored cadavers of Protaetia brevitarsis seulensis larvae recovered from soil samples taken from Gwangju, Gyeonggi-do Province, Republic of Korea. Soil samples were collected from a mountainside dominated by acutissima oak (Quercus acutissima Carr.) trees. The nematodes were recovered from the insect cadavers using the white trap method (White, 1927). Females, males, and dauer juveniles of P. koreana n. sp. were handpicked from the nematode suspension under a Nikon SMZ 1000 stereomicroscope (Nikon, Nikon Corporation Tokyo, Japan). The population was subsequently characterized based on inferences from DNA barcodes and morphometric data. Also, being a predominantly gastropod-parasitic genus, an additional preliminary test to confirm entomo-parasitic relationship was conducted on the larval stage of Spodoptera exigua. The susceptibility of first and second-instar larvae of S. exigua was tested in Petri dishes (9 cm diameter) lined with a 7 cm diameter Whatman No. 1 filter paper disk. Dauer juveniles of P. koreana n. sp. were applied onto the filter paper at rates of 50, 100, and 150 dauer juveniles per larva. Four larvae of each instar were gently transferred to the Petri dish and incubated at 25°C. Dauer juveniles were not added to the control, and larvae were not fed during the treatment. Larval mortality was recorded 24 h, 48 h, and 72 h after application.
The nematodes were heat-killed and fixed with hot formalin-glycerin proportion and processed to pure glycerin according to Seinhorst (1959) as modified by De Grisse (1969). The processed specimens were mounted on permanent slides and examined under a fluorescence microscope. Photomicrographs and measurement data were taken using a Zeiss imager Z2 microscope (Carl Zeiss) fitted with Axio-vision, a material science software for research and engineering (Carl Zeiss). Line drawings were made under a drawing tube and redrawn using CorelDRAW® software version 24 (Corel Corporation Ottawa, Canada). Species diagnosis was done following original species descriptions and comparing morphometric data with the closest species of the genus.
Genomic DNA was extracted from heat-relaxed morphometrically confirmed female and male specimens using the DNA extraction kit WizPure™ (Wizbiosolutions Inc. Seongnam, South Korea) according to Iwahori et al. (2000). Four gene fragments, i.e., 18S-rRNA gene, the D2–D3 expansion segment of 28S-rRNA gene, the partial ITS-rRNA gene, and the partial COI gene, were amplified and sequenced in this study. The two primer sets: 988F (5′-CTCAAAGATTAAGCCATGC-3′) and 1912R (5′-TTTACGGTCAGAACTAGGG-3′), and 1813F (5′-CTGCGTGAGAGGTGAAAT-3′) and 2646R (5′-GCTACCTTGTTACGACTTTT-3′) (Holterman et al., 2006) were used to amplify the nearly full-length 18S-rRNA gene, as two partially overlapping fragments. The primer set D2A (5′-ACAAGTACCGTGAGGGAAAGTTG-3′) and D3B (5′-TCGGAAGGAACCAGCTACTA-3′) (Nunn, 1992) amplified the D2–D3 expansion segment of 28S-rRNA gene; Vrain2F (5′-CTTTGTACACACCGCCCGTCGCT-3′) and Vrain2R (5′-TTTCACTCGCCGTTACTAAGGGAATC-3′) (Vrain et al., 1992) amplified the partial ITS-rRNA gene; and the partial COI gene was amplified using the primer set COIF1 (5′-CCTACTATGATTGGTGGTTTTGGTAATTG-3′) and COIR2 (5′-GTAGCAGCAGTAAAATAAGCACG-3′) (Kanzaki and Futai, 2002). Polymerase chain reaction (PCR) was performed with a PCR cycler (T100™, Bio-Rad Hercules, USA). The thermal cycle for the primer sets, D2A/D3B, 988F/1912R, 1813F/2646R, and COIF1/COIR2, was as described by Mwamula et al. (2023), and the thermal profile for Vrain2F/Vrain2R primer set was set as follows: initial denaturation at 95°C for 5 min, 35 cycles at 95°C for 30 s, followed by an annealing step at 53°C for 30 s; 72°C for 1 min, and finally one cycle at 72°C for 10 min. The products were purified using the QIAquick PCR Purification Kit (Qiagen Hilden, Germany) and quantified using a QuickDrop spectrophotometer (Molecular Devices San Jose, USA). The purified products were directly sequenced with the primers specified above at Macrogen Inc. (Seoul, South Korea). The new sequences were edited and submitted to the NCBI GenBank database under the accession numbers: PV031561, PV031562 (for 18S-rRNA); PV031559, PV031560 (for 28S-rRNA); PV031557, PV031558 (for ITS-rRNA); and PV031884, PV031885 (for COI gene).
Using the BLAST homology search program, the obtained sequences (18S-rRNA, 28S-rRNA, ITS-rRNA, and COI gene) were compared with those of related species of Pellioditis, including comparable sequences of species from other related genera published in GenBank (Huang et al., 2015; Nermuť et al., 2016a, 2016b, 2017; Tandingan De Ley et al., 2016; Ivanova and Spiridonov, 2017, 2023; Ivanova et al., 2020, 2022; Pieterse et al., 2020; Zhang and Liu, 2020; Gorgadze et al., 2022). Multiple alignments for the selected genes (18S-rRNA, 28S-rRNA, and ITS-rRNA) were built using ClustalX (Thompson et al., 1997). The sequences of Oscheius citri Tabassum et al., 2016 (MK932670), Litoditis mediterranea (Sudhaus, 1974) Sudhaus, 2011 (AF083020), and Litoditis marina (Bastian, 1865) Sudhaus, 2011 (AF083021) were used as the outgroup taxa for the 18S-rRNA gene; L. mediterranea (EU195973), Rhabditella axei (Cobbold, 1884) Chitwood, 1933 (AY602177), and Oscheius chongmingensis (Zhang et al., 2008) (MT548600) were the outgroup taxa for 28S-rRNA gene; and Oscheius tipulae (Lam and Webster, 1971) (JF920965), Oscheius onirici Torrini et al., 2015 (KX036751), and Heterorhabditis georgiana Nguyen et al., 2008 (KY568701) were selected as the outgroup taxa for ITS-rRNA gene. Bayesian inference (BI) of the phylogenies was performed using MrBayes 3.2.7 (Ronquist et al., 2012), with GTR + I + G model selected for all three datasets. BI analysis was initiated with a random starting tree and run with four chains for 1 × 106 generations. The Markov chains were sampled at intervals of 100 generations. Consensus trees were generated with the 50% majority rule. The generated trees were edited using FigTree v1.4.4 software (http://tree.bio.ed.ac.uk/software/figtree/) (Rambaut, 2018). Posterior probabilities (PP) exceeding 50% are given on appropriate clades. Intraspecific and interspecific sequence variation was analyzed using PAUP* v4.0a169 (Sunderland, MA, USA: Sinauer Associates) (Swofford, 2003).
Pellioditis koreana n. sp. (Figs. 1–4)
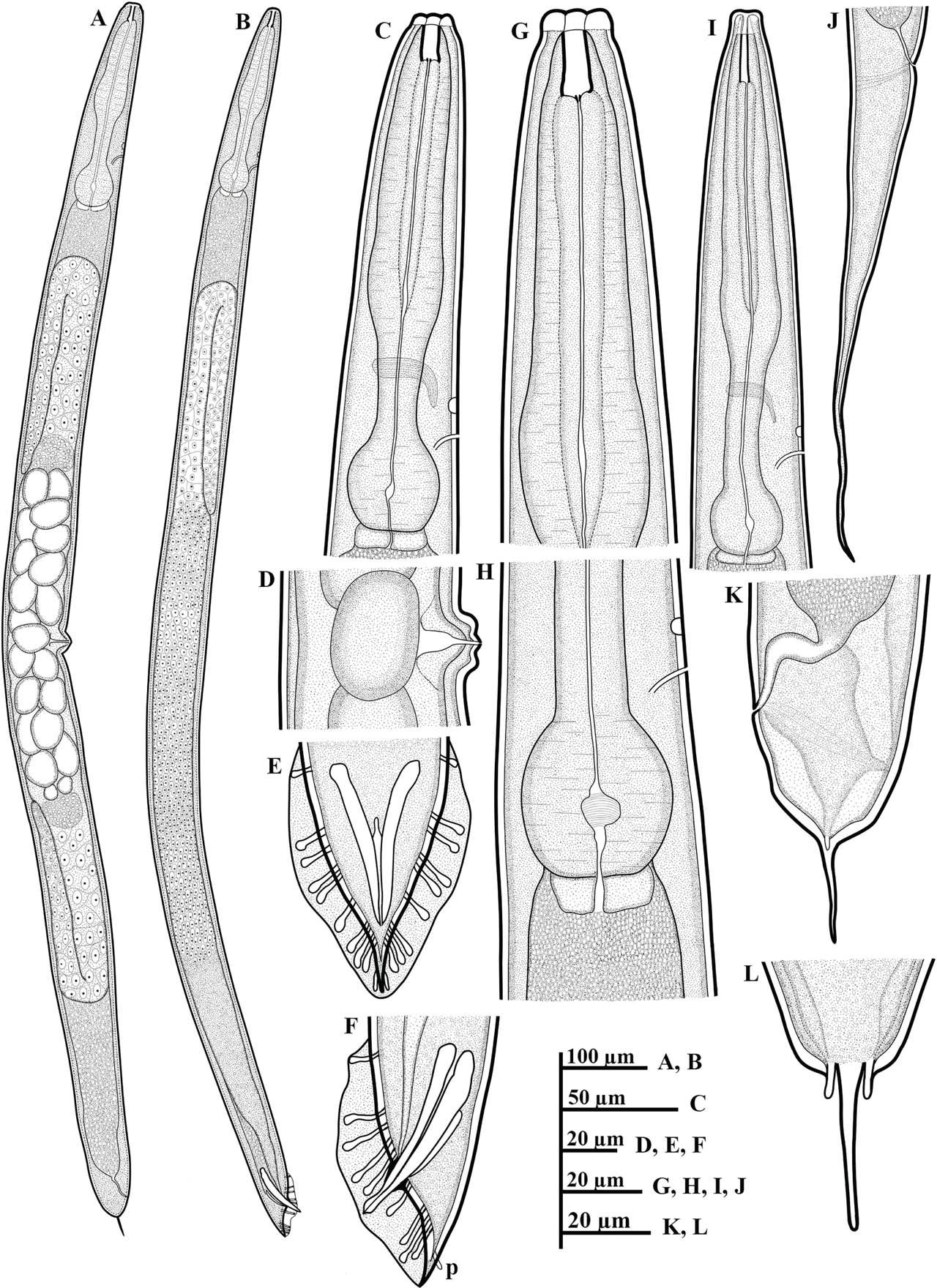
Line drawings of P. koreana n. sp. (A–L): (A) female whole body; (B) male whole body; (C,G) female anterior region; (D) vulval region; (E,F) posterior region of male, including copulatory apparatus and the arrangement of GP (p = phasmid); (H) basal bulb region; (I) anterior region of dauer juvenile; (J) tail region of dauer juvenile; and (K,L) female tail region including the shape of phasmids. GP, genital papillae.
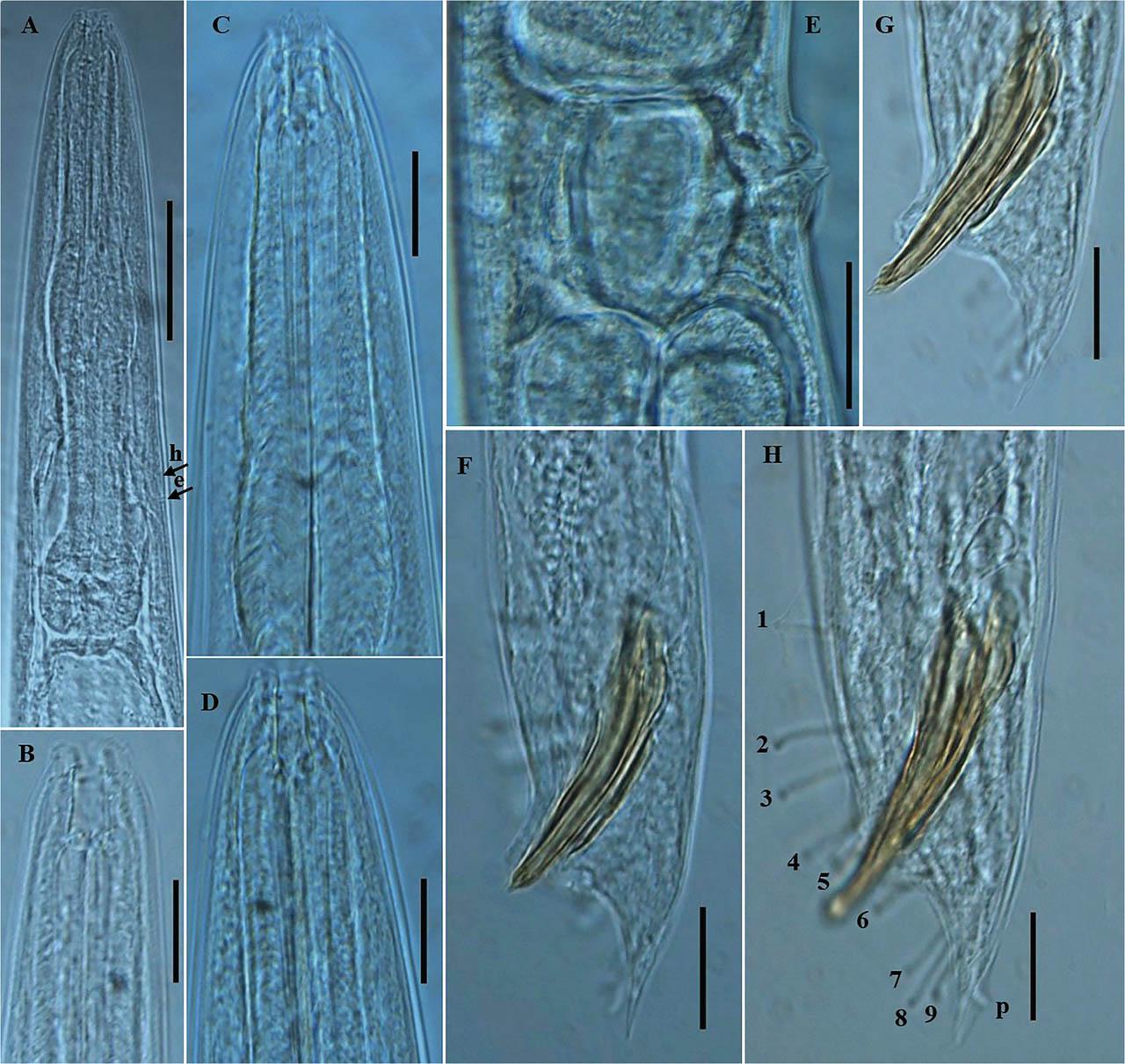
Photomicrographs of P. koreana n. sp. (A–H). (A–D) Female anterior region; (E) vulval region; and (F–H) posterior region of male, including copulatory apparatus and the arrangement of GP. The arrows labeled h and e indicate the position of hemizonid and excretory pore, respectively; p = phasmid (Scale bars: A = 50 μm; B–H = 20 μm). GP, genital papillae.
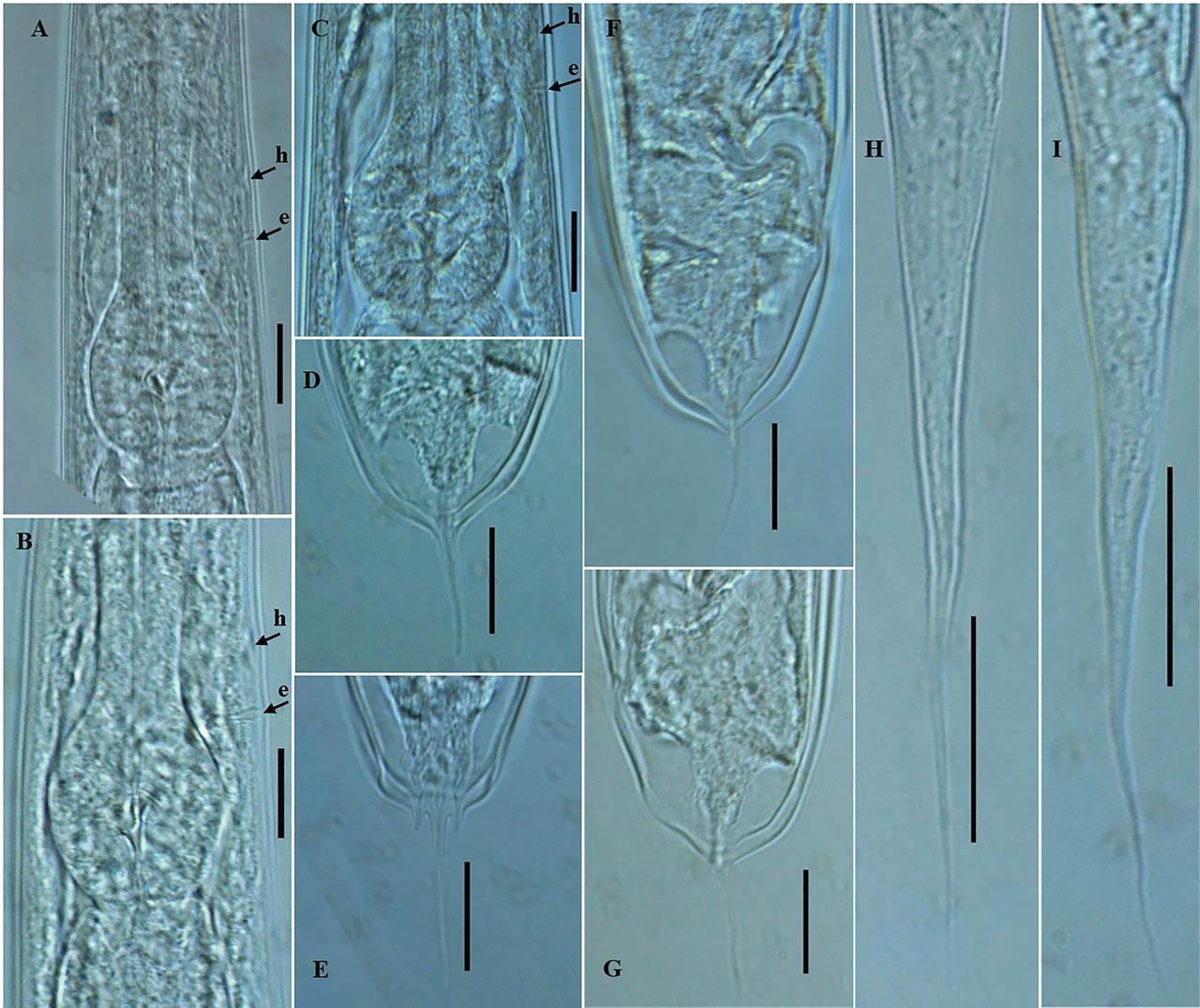
Photomicrographs of P. koreana n. sp. (A–I). (A–C) Posterior region of the pharynx; (D,F,G) female tail region, (E) caudal part with prominent phasmids; and (H,I) dauer juvenile tail. The arrows labeled h and e indicate the position of hemizonid and excretory pore, respectively, (scale bars: A–G = 20 μm; H,I = 30 μm).
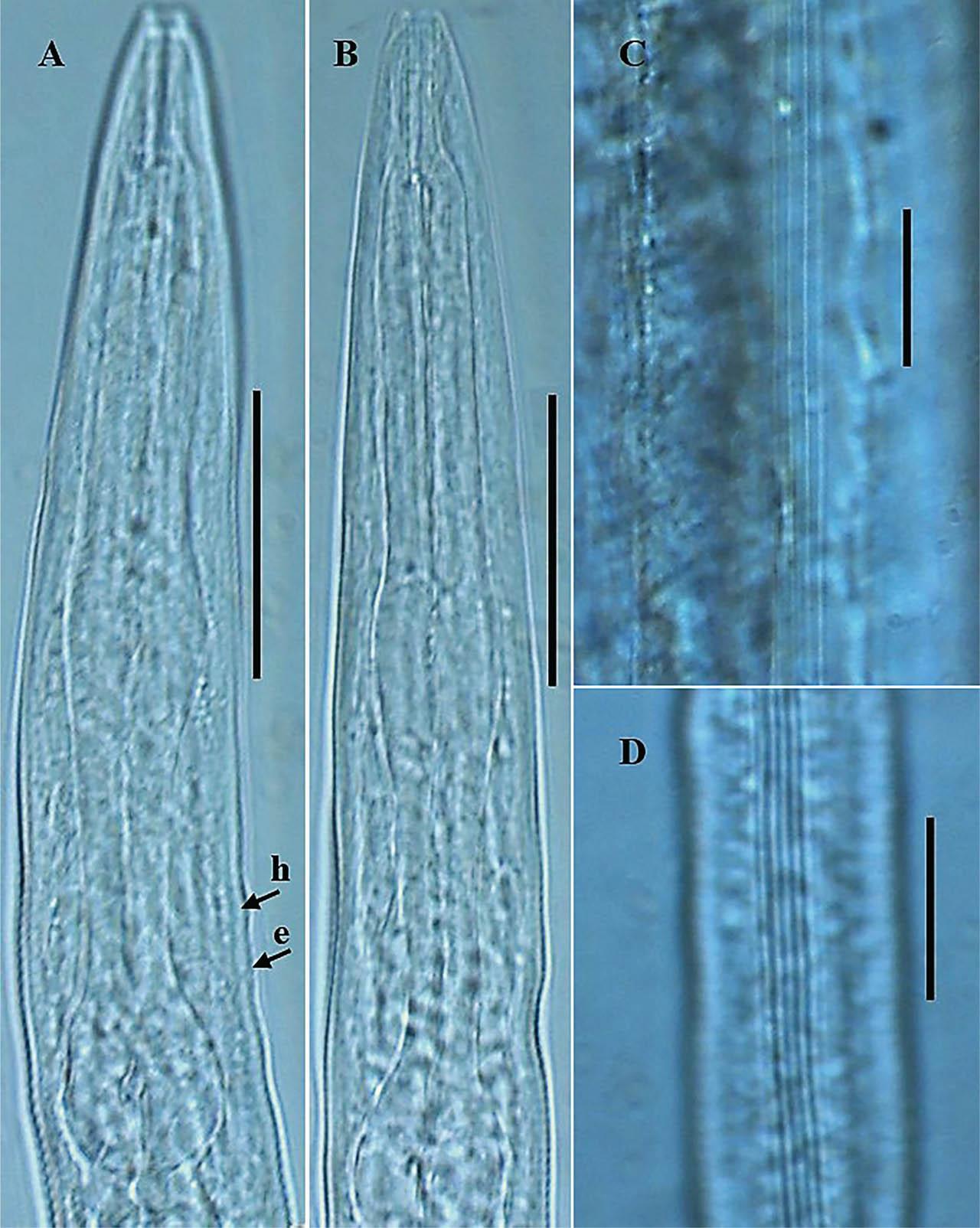
Photomicrographs of P. koreana n. sp. (A–D). (A,B): Dauer juvenile anterior region; (C) lateral field in female; and (D) lateral field in dauer juvenile. The arrows labeled h and e indicate the position of the hemizonid and excretory pore, respectively, (scale bars: A,B = 30 μm; C,D = 20 μm).
See Table 1.
Morphometrics of P. koreana n. sp. from Korea.
| Character | Holotype ♀ | ♀♀ | ♂♂ | Dauer juveniles |
|---|---|---|---|---|
| n | 20 | 20 | 20 | |
| L | 1,530.0 | 1,520.0 ± 116.0 (1,265.0–1,734.0) | 1,181.4 ± 104.1 (998.0–1,374.0) | 585.9 ± 38.2 (517.0–643.0) |
| a | 18.1 | 19.7 ± 1.4 (17.2–23.7) | 22.0 ± 1.0 (20.4–23.6) | 22.9 ± 1.6 (20.7–26.8) |
| b | 6.6 | 6.8 ± 0.5 (5.7–7.4) | 6.1 ± 0.6 (4.3–6.7) | 4.5 ± 0.2 (4.1–4.7) |
| c | 29.1 | 27.4 ± 2.5 (23.3–31.0) | 34.8 ± 2.8 (30.0–39.3) | 4.9 ± 0.3 (4.4–5.4) |
| c' | 1.3 | 1.4 ± 0.1 (1.2–1.6) | 1.4 ± 0.1 (1.1–1.5) | 7.8 ± 0.7 (6.7–9.8) |
| V or T | 52.7 | 52.3 ± 1.0 (50.9–54.5) | 71.1 ± 2.9 (64.8–75.2) | – |
| G1% | 29.8 | 29.4 ± 2.8 (24.9–35.4) | – | – |
| G2% | 28.7 | 29.2 ± 3.0 (22.3–33.8) | – | – |
| Lip height | 6.5 | 5.1 ± 0.7 (4.0–6.5) | 5.1 ± 0.7 (3.0–6.5) | 2.0 ± 0.2 (1.5–2.5) |
| Lip diam. | 14.0 | 15.6 ± 0.9 (13.5–16.5) | 14.2 ± 1.0 (12.0–15.5) | 5.2 ± 0.5 (4.5–6.5) |
| Stoma length | 20.5 | 20.1 ± 0.5 (19.0–21.0) | 19.3 ± 1.3 (16.5–21.0) | 16.6 ± 0.9 (14.5–18.0) |
| Stoma diam. | 5.5 | 5.8 ± 0.5 (4.5–7.0) | 5.0 ± 0.7 (4.0–6.5) | – |
| Corpus length | 134.0 | 130.2 ± 4.8 (120.0–138.0) | 113.3 ± 5.1 (103.5–123.0) | 79.4 ± 5.1 (64.0–89.0) |
| Metacarpal diam | 29.0 | 30.2 ± 1.7 (26.0–34.0) | 26.7 ± 1.7 (23.0–29.5) | 13.7 ± 1.4 (10.5–16.0) |
| Isthmus length | 59.5 | 53.3 ± 5.1 (44.5–62.5) | 45.4 ± 3.6 (40.0–52.0) | 31.5 ± 2.6 (24.5–35.0) |
| Basal bulb length | 40.0 | 39.5 ± 1.6 (37.0–43.5) | 33.9 ± 2.9 (29.5–40.5) | 18.0 ± 1.3 (14.0–20.0) |
| Basal bulb diam. | 39.5 | 37.2 ± 2.5 (32.5–41.5) | 30.5 ± 1.9 (27.0–33.5) | 16.3 ± 1.0 (14.0–18.5) |
| Cardia length | 12.8 | 9.8 ± 2.9 (6.5–15.0) | 7.6 ± 1.8 (6.0–12.5) | 3.4 ± 0.4 (3.0–4.0) |
| Anterior end to nerve ring | 151.5 | 145.5 ± 6.2 (134.0–161.0) | 128.6 ± 8.0 (115.5–151.0) | 90.3 ± 6.8 (72.0–107.0) |
| Anterior end to hemizonid | 183.0 | 177.6 ± 9.2 (160–191.0) | 155.3 ± 8.9 (136.5–169.0) | 107.2 ± 8.1 (88.0–119.0) |
| Anterior end to Ex. pore | 198.0 | 191.8 ± 11.4 (171.0–206.0) | 170.6 ± 10.2 (148.5–185.5) | 115.3 ± 7.7 (99.0–129.0) |
| Pharynx length | 232.0 | 224.7 ± 7.3 (212.0–239.0) | 194.8 ± 12.7 (175.5–234.5) | 130.8 ± 6.2 (121.5–145.0) |
| Vulval body diam. | 84.5 | 76.9 ± 7.5 (63.0–88.0) | – | – |
| Maximum body diam. | 86.0 | 77.6 ± 7.8 (63.0–88.5) | 53.9 ± 4.9 (44.5–63.0) | 25.6 ± 2.1 (22.0–30.0) |
| Rectum | 39.5 | 40.6 ± 4.0 (34.0–49.0) | – | 20.8 ± 2.0 (17.0–24.0) |
| Anal/cloacal body diam. | 42.0 | 41.2 ± 4.3 (34.5–50.5) | 25.2 ± 3.0 (22.0–32.5) | 15.4 ± 1.4 (11.5–17.5) |
| Tail length | 52.5 | 55.7 ± 4.7 (46.0–62.5) | 34.0 ± 2.5 (30.0–39.0) | 118.9 ± 6.3 (112.0–132.0) |
| Tail spike length | 21.5 | 25.3 ± 4.3 (17.0–38.0) | – | |
| Spicules | – | – | 55.2 ± 5.6 (48.0–70.5) | – |
| Gubernaculum | 23.6 ± 1.8 (20.5–28.0) | |||
All measurements are in μm and in the form: average ± SD (range).
Body robust, generally straight to slightly ventrally arcuate in the middle when heat-killed and fixed. Cuticle with fine annules. Lateral fields with three ridges and four lines. Lip region continuous with body, 2.2–3.8 times as wide as high, slightly flattened anteriorly with six visible lips bearing two circles of sensilla, each lip with a terminal labial papilla. Stoma 3–4 times as long as wide, with cheilostome not cuticularized. Gymnostom walls parallel, thickened. Stegostom with glottoid apparatus, metarhabdions thickened, with two wart-like denticles (Fig. 2B). Pharynx muscular, consisting of a corpus with a slightly swollen non-valvular metacarpal region, a slender isthmus, and a swollen, round, or bulbous terminal bulb with pronounced striated valvular apparatus. Cardia present, variable in length. Nerve ring encircling the slender isthmus at 61%–70% of the total pharynx length from the anterior end. Anterior part of the pharynx (procorpus + metacorpus) 1.2–1.6 times longer than the posterior part (isthmus + terminal bulb). Hemizonid clearly visible, ca 5 μm long, located adjacent to the middle or posterior part of the isthmus. Excretory pore prominent and with a well-cuticularized duct, located anterior to the basal bulb or within the region of the anterior portion of the basal bulb, 9.0–21.0 μm posterior to the hemizonid. The reproductive system didelphic amphidelphic, well developed with reflexed ovaries. Anterior and posterior branches of nearly similar length, filled with spherical-shaped oocytes. Spermatheca present, more visible in less gravid females, often filled with numerous sperm. Uteri of mature females filled with spherical-shaped oocytes, commonly hatching inside the body. Vagina perpendicular to body axis, its length variable, less than half of vulval body diam. in gravid females but often extending past the middle of the vulval body diam. in less gravid females. The vulva a closed transverse slit with protruding lips, located at the midbody region. The digestive system simple. Rectum prominent, wide, and curled along its length. Anus an arcuate slit. Anal body diam. 63.5%–81% of tail length. Tail with the anterior wider cupola-shaped portion, with angular sides, terminating into a moderately slender pointed spike, forming 34.5%–61.0% of the tail length. Phasmids prominent, papilla-like, flanking the base of the angular cupola part of the tail.
Generally, as abundant as females. General morphology similar to that of females except for sexual characters, papillae, and a conical, pointed tail. Body straight to slightly ventrally arcuate, body generally shorter and smaller than female. Hemizonid and excretory pore prominent, positioned as in females. Testis reflexed, occupying 65%–75% of the body length. The germinal part of the testis ends in a blunt tip. Spermatogonia arranged in 2–3 rows; maturing spermatocytes located proximally in 2–3 rows. Vas deferens wide, filled with a large number of sperm cells. Caudal region surrounded by an open peloderan bursa, lined with nine pairs of genital papillae (GP) or bursal rays with a typical arrangement of 1 + (1 + 1) + 2 + 1 + 3 (Fig. 1E,F; 2H). In the lateral view, the first three pairs of GP (GP1, GP2, and GP3) precloacally located, with GP1 located far anteriorly from GP2 and GP3. GP2 and GP3 spaced closer to each other than to the ensuing pair (GP4 and GP5). GP4 and GP5 standing as a pair in an adcloacal position; GP6 to GP9 located post-cloacally. GP6 positioned at mid position between the preceding pair (GP4 and GP5) and the ensuing trio (GP7–GP9). GP7, GP8, and GP9 located close to each other. GP3, GP4, and GP8 are the longest, ending just short distance to the bursal edge; all other papillae shorter and do not reach the edge of the bursa. Phasmids papilla-like, located posterior to GP9 (Figs. 1E,F; 2H). Spicules paired, slightly arcuate on the ventral side, and with light swelling just before the pointed tip. Gubernaculum weakly arched, less than half (33%–48%) of spicule length long. Tail short conoid, tapering to a pointed ventrally arcuate terminus.
General body habitus straight to slightly arcuate in the tail region when heat-killed, slender, tapering towards the head and tail end regions. Cuticle with a tessellated appearance, the outer sheath of cuticle fitted more tightly to both body ends, mouth aperture not enclosed. Lateral field with five closely spaced ridges and six lines on each side. Lip region flat, continuous with body contour, 2.3–3.5 times as wide as high. Stoma long and thin (or 7.4–13.5 times as wide as long), with cheilostom not cuticularized. Pharyngeal structure typical for the genus. Hemizonid and excretory pore visible. Hemizonid located adjacent to the middle or posterior part of the isthmus, excretory pore located anterior to the basal bulb or within the region of the anterior portion of the basal bulb, 4.0–12.0 μm posterior to hemizonid. Nerve ring encircling the slender isthmus in the anterior or mid portion. Cardia short. The rectum widens and curls along its length. Tail long, conical, and tapering to a filiform posterior end. Phasmids visible, situated 25.0–29.0 μm posterior to anus.
Pellioditis koreana n. sp. is characterized by its moderately robust body, 1.27–1.73 mm long, lip region 2.2–3.8 times as wide as high, lateral fields with three ridges and four lines, pharynx with rounded basal bulb, anterior part of the pharynx 1.2–1.6 times longer than posterior part, hemizonid prominent, clearly visible, located adjacent to the middle or posterior part of isthmus, excretory pore located anterior to basal bulb or within the region of the anterior portion of basal bulb, 9.0–21.0 μm posterior to hemizonid, vulva located at midbody region (51.0%–54.5%), tail cupola-shaped, with angular sides, terminating into a slender pointed spike, phasmids prominent, papilla-like, flanking the base of the angular cupola part of the tail, male caudal region surrounded by an open peloderan bursa, with nine pairs of GP with a typical arrangement of 1 + (1 + 1) + 2 + 1 + 3, spicules paired, 48.0–70.5 μm long, gubernaculum less than half of spicule length long, dauer juvenile with a long tail, conical, and tapering to a filiform posterior end.
By having a cupola-shaped tail flanked by a prominent papilla-like pointed spike, P. koreana n. sp. belongs to the Papillosa group and closely resembles P. zhejiangensis in both morphometric and molecular DNA barcode data. However, P. koreana n. sp. can be differentiated from P. zhejiangensis by its less robust body (L = 1.27–1.73 mm vs. 1.44–2.35 mm, and a = 17.2–23.7 vs. 14.1–18.8), excretory pore located anterior to basal bulb or within the region of the anterior portion of basal bulb vs. posterior to basal bulb, shorter cupola-shaped tail, with angular sides (46.0–62.5 μm vs. 60.0–83.5 μm), GP arrangement of 1 + (1 + 1) + 2 + 1 + 3 vs. 1 + 1 + 1 + 2 + 1 + 3, and all morphometric measurements of dauer juveniles except L (a = 20.4–23.6 vs. 16.1–18.9, c = 4.4–5.4 vs. 5.6–9.4, c’ = 6.7–9.8 vs. 1.1–1.5, and tail length 112–132.0 μm vs. 69.4–92.2 μm). P. koreana n. sp. is also comparable to five other members of the genus with cupola-shaped tail, including Phasmarhabditis papillosa (Schneider, 1866) Andrássy (1976), Phasmarhabditis safricana Ross et al., 2018, P. bonaquaense Nermuť et al., 2016b, P. meridionalis Ivanova and Spiridonov, 2017, and P. huizhouensis Huang et al., 2015. P. koreana n. sp. differs from P. papillosa by the short tail length (46.0–62.5 μm vs. 73.0–130.0 μm), with a short tail spike (17.0–38.0 μm vs. 51.0–82.0 μm), c = 23.3–31.0 vs. 10.9–26.3, c' = 1.2–1.6 vs. 1.9–3.6, prominent papilliform phasmids flanking the base of the angular cupola part of the tail vs. barely visible, and male GP arrangement (see Pieterse et al. (2017) for arrangement in P. papillosa). The new species differs from P. safricana by prominent papilliform phasmids vs. smaller, non-projecting phasmids, GP arrangement of 1 + (1 + 1) + 2 + 1 + 3 vs. 1 + 1 + 1 + 2/1 + 3 formulae, dauer juvenile morphometrics including head with no dorsal tooth vs. a single dorsal tooth present, and long tail length (112–132.0 μm vs. 52.0–60.0 μm, c′ = 1.2–1.6 vs. 8.7–9.8).
Pellioditis koreana n. sp. differs from P. bonaquaense by its less robust body (females: L = 1,265.0–1,734.0 μm vs. 1,878–2,626 μm, a ratio = 17.2–23.7 vs. 13.3–21.7; males: L = 998.0–1,374.0 μm vs. 1,414–2,121 μm), shorter tail length (females: 46.0–62.5 μm vs. 66.5–109.5 μm; males 30.0–39.0 μm vs. 47.0–54.5 μm), shorter spicules (48.0–70.5 μm vs. 68.5–86.0 μm), shorter gubernaculum (20.5–28.0 μm vs. 28.5–40.0 μm), GP arrangement of 1 + (1 + 1) + 2 + 1 + 3 vs. 1 + 1 + 1 + 2 + 1 + 3, shorter body length in dauer juveniles (517.0–643.0 μm vs. 808–1,050 μm), long filiform tail in dauer juveniles (112–132.0 μm) vs. a shorter tail with a blunt tip (71.5–102.0 μm). P. koreana n. sp. differs from P. meridionalis by the short spicules (48.0–70.5 μm vs. 71.0–83.0 μm), short gubernaculum (20.5–28.0 μm vs. 40.0–46.0 μm) prominent papilliform phasmids in females, flanking the base of the angular cupola part of the tail vs. less projecting and thin phasmids, tail with a moderately slender pointed spike (17.0–38.0 μm long) vs. a long filamentous thin spike (27.0–50.0 μm long), pharynx with rounded basal bulb vs. pear-shaped, vulva with protruding lips vs. flat lips, and shorter body length in dauer juveniles (517.0–643.0 μm vs. 770.0–912.0 μm). Finally, the new species differ from P. huizhouensis by a slender body (max body diam. 63.0–88.5 μm vs. 85.5–171.0 μm; a = 17.2–23.7 vs. 12.6–16.0), shorter angular cupola-shaped tail (46.0–62.5 μm vs. 81.0–105.5 μm), shorter tail spike (17.0–38.0 μm vs. 48.5–64.0 μm), c = 23.3–31.0 vs. 15.5–26.9, shorter male tail (30.0–39.0 μm vs. 35.5–61.5 μm), GP arrangement of 1 + (1 + 1) + 2 + 1 + 3 vs. 1 + 1 + 1 + 2 + 1 + 3 formulae, and short gubernaculum (20.5–28.0 μm vs. 30.0–41.0 μm). It is also important to note that the hemizonid has not been observed (or described) in almost all the known nominal species of the genus. However, in P. koreana n. sp., the hemizonid and excretory pore are prominent and clearly visible in both sexes.
The nematode population was extracted from brown-colored cadavers of Protaetia brevitarsis seulensis larvae recovered from soil samples taken from Gwangju, Gyeonggi-do Province, Republic of Korea (GPS coordinates: 37°27′10.67″N, 127°17′14.5″E).
Holotype female, 13 females, 12 males, and 15 dauer juvenile paratypes were deposited in the National Institute of Biological Resources of Korea, and 6 females, 8 males, and 5 dauer juvenile paratypes were deposited in the Nematode Collection of Kyungpook National University (KNU), Republic of Korea.
Pellioditis koreana n. sp. was isolated and described from the Korean Peninsula region. Thus, the species epithet koreana is derived from the name of the country of its first description, i.e., Korea.
The amplified nearly full-length 18S-rRNA, the partial 28S-rRNA gene, and ITS-rRNA gene, and the COI gene yielded fragments of approximately 1,700 bp, 680 bp, 990 bp, and 630 bp, respectively. The two obtained 18S-rRNA gene partial sequences (PV031561 and PV031562) were identical with no recorded intraspecific variation. In the 18S-rRNA gene phylogeny, P. koreana n. sp. sequences were grouped in a moderately supported clade with the closely homologous sequences of P. zhejiangensis (MT371565), Pellioditis sp. (MG252037), Pellioditis thesamica (OU753546), Pellioditis bohemica (KX017478, KX017479), Angiostoma namekuji (MF838864) and P. huizhouensis (KP017252), differing by 24–27 bp (2.7%–3.0%), 20–24 bp (1.3%–1.5%), 32–40 bp (2.1%–2.5%), 24–33 bp (1.5%–2.0%), 37–39 bp (2.4%) and 49–53 bp (3.2%–3.3%), respectively. The two D2–D3 sequences of P. koreana n. sp. (PV031559, PV031560) were also identical, with no intraspecific sequence variation (0.0%), and were also almost identical to P. zhejiangensis (MK937096), differing by only 3 bp (0.4%). Based on the 28S-rRNA gene phylogeny, the sequences of P. koreana n. sp. were also grouped in a well-supported subclade (PP = 100%) with other sequences of Pellioditis spp., including Pellioditis sp. (PQ810652), Pellioditis sp. (PQ738941), Pellioditis sp. (PQ810653), P. clausiliiae (MK500248), and P. thesamica (OU753547), differing by 2 bp (0.3%), 14 bp (2.3%), 17 bp (2.8%), 18 bp (3.2%), and 25 bp (4.2%), respectively.
The two partial ITS-rRNA gene sequences of P. koreana n. sp. (PV031557, PV031558) were grouped in a separate well-supported clade with sequences of P. zhejiangensis (MK542667) and unidentified Pellioditis sp. (PQ738940), differing by 20 bp (2.6%) and 76 bp (8.7%), respectively. Owing to the unavailability of COI gene sequences for the genus in the GenBank database, the generated sequences for P. koreana n. sp. (PV031884, PV031885) only showed relative homology with COI gene sequences of Pellioditis sp. (MF167646), with percent identities of 89.8% based on the BLAST homology search program. Thirty-nine 18S-rRNA gene-, 72 28S-rRNA gene-, and 45 ITS-rRNA gene sequences from member species of Pellioditis, and other related genera, including the newly obtained sequences and outgroup taxa, constituted the sequence dataset for phylogenetic analyses. Phylogenetic relationships, as inferred from Bayesian analysis of the dataset with the GTR + I + G substitution model, are shown in Figures 5–7.
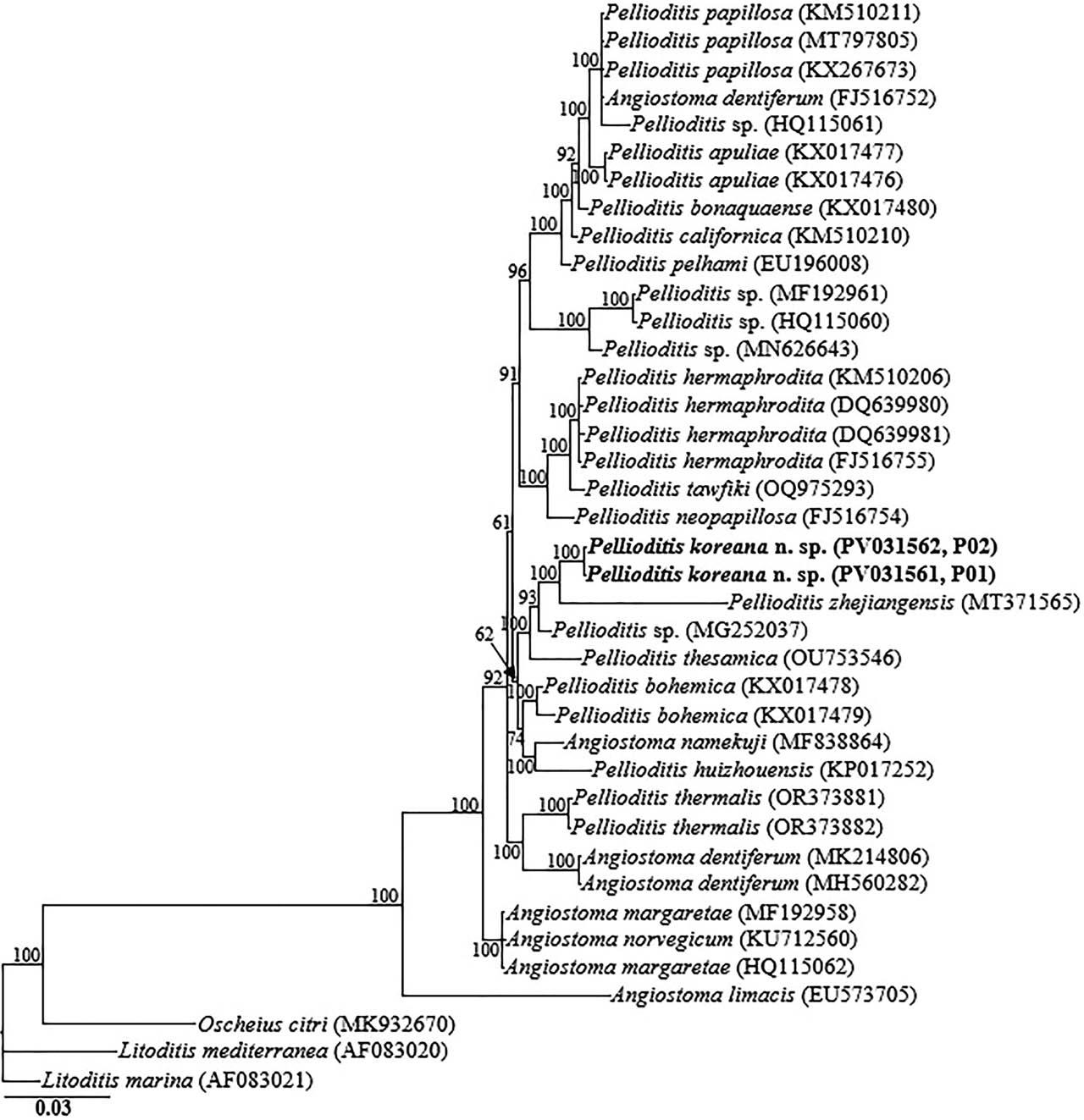
Bayesian tree inferred under the GTR + I + G model from 18S-rRNA gene sequences of Rhabditid species. Posterior probability values exceeding 50% are given on appropriate clades. The studied population is indicated in bold text. Outgroup taxa: O. citri, L. mediterranea and L. marina.
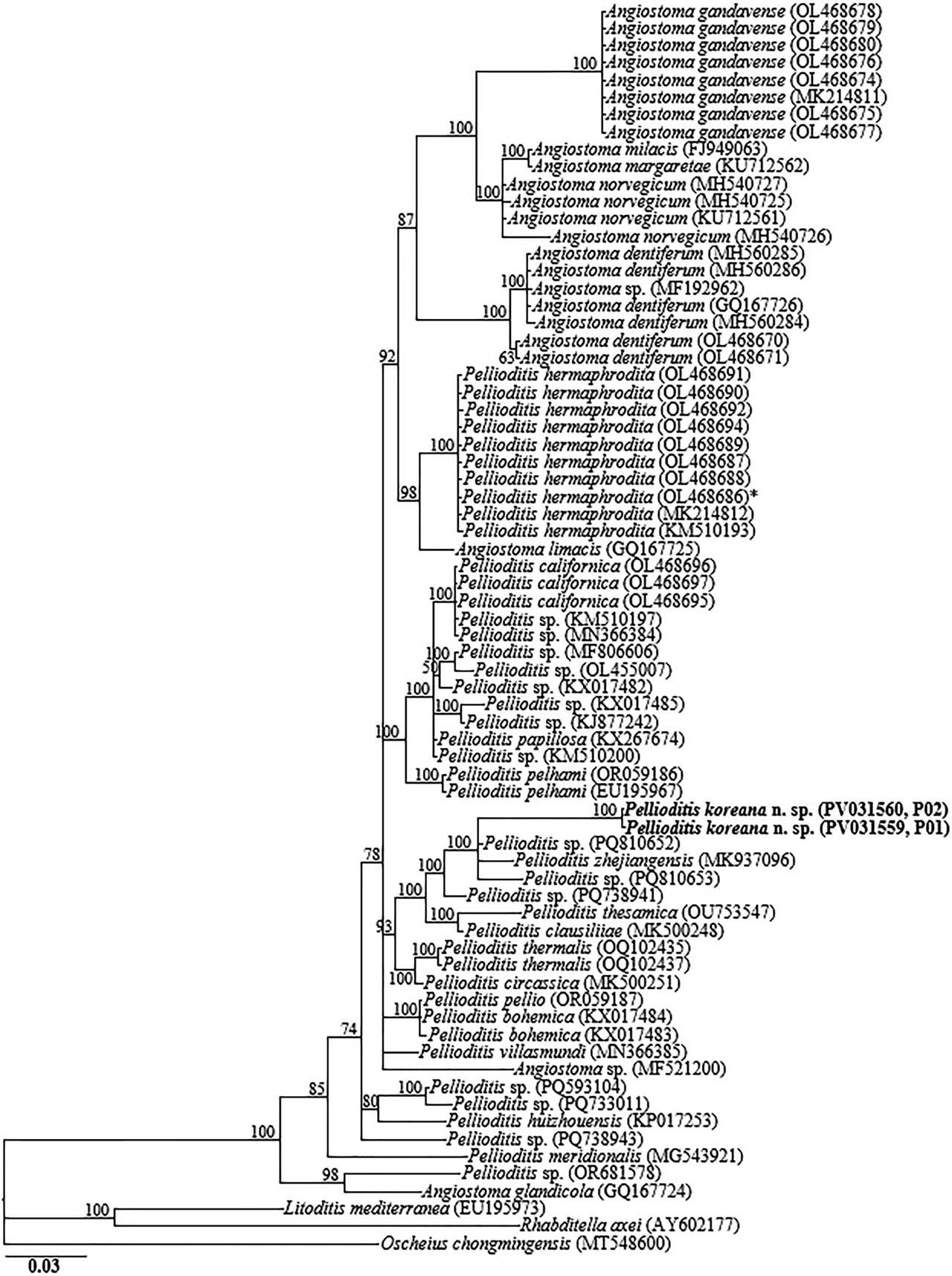
Bayesian tree inferred under the GTR + I + G model from LSU D2–D3 partial sequences of Rhabditid species. Posterior probability values exceeding 50% are given on appropriate clades. The studied population is indicated in bold text. Outgroup taxa: L. mediterranea, R. axei and O. chongmingensis.
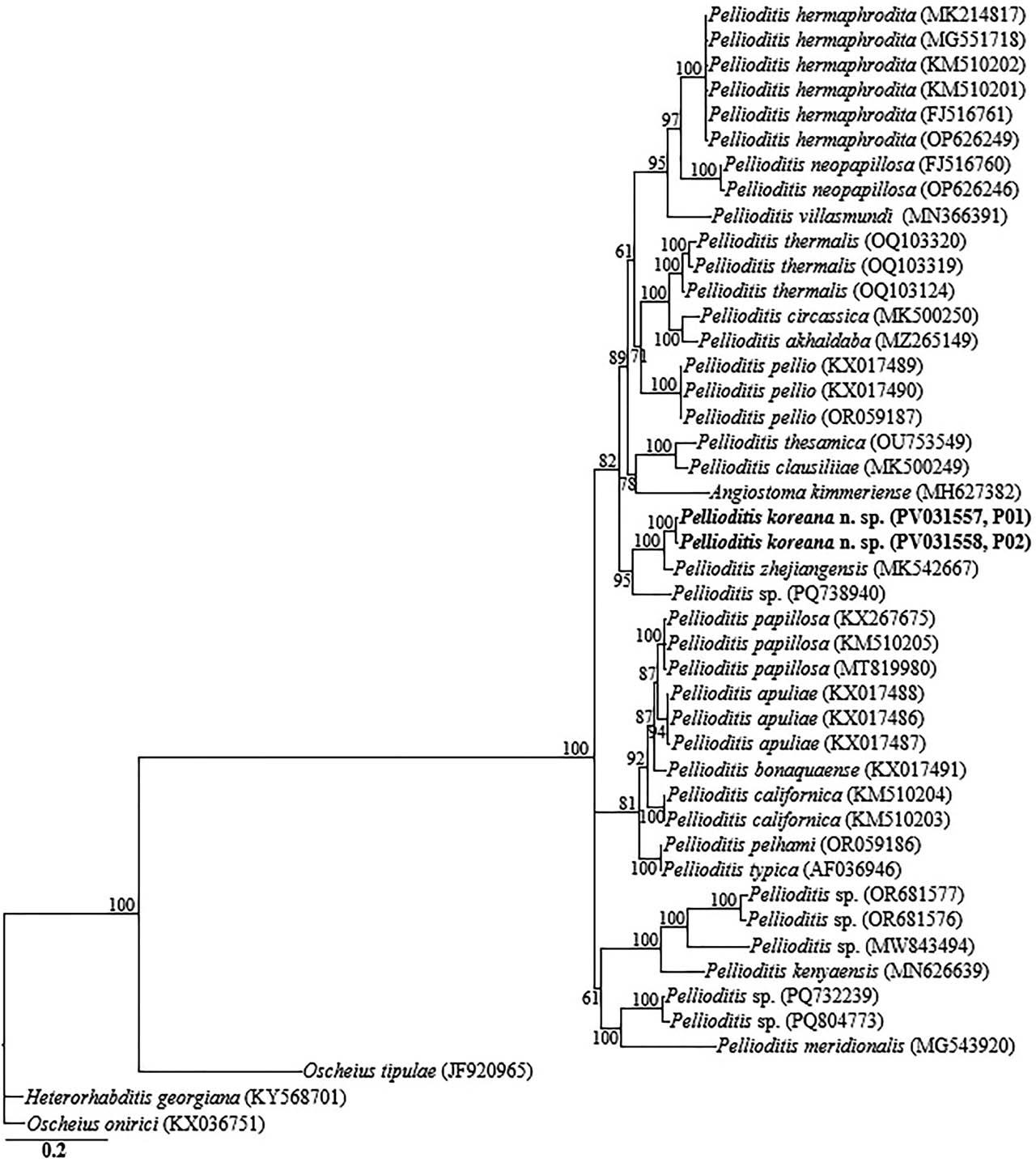
Bayesian tree inferred under the GTR + I + G model from ITS-rRNA partial sequences of Rhabditid species. Posterior probability values exceeding 50% are given on appropriate clades. The studied population is indicated in bold text. Outgroup taxa: O. tipulae, O. onirici and H. georgiana.
Inferences from the phylogenetic analyses of the three genes, especially the partial 18S-rRNA gene and partial ITS-rRNA gene sequences, suggest that P. koreana n. sp. is genetically distinct from the available Pellioditis gene sequences as represented by the Bayesian trees. The inferences suggest that P. koreana n. sp. is a cryptic sister species to the morphologically close P. zhejiangensis. Pellioditis is a stenomorphic genus, with very few unique and reliable morphological characters to aid species delineation due to the conservative body morphology (Nermuť et al., 2016a; Tandingan De Ley et al., 2016; Ivanova and Spiridonov, 2017; Ivanova et al., 2020). This is even exacerbated by the significant morphometric variations recorded within the same species depending on maintenance conditions of the isolates (in vivo vs. in vitro) (Hooper et al., 1999; Pieterse et al., 2017).
DNA barcoding is a powerful tool for nematode identification, especially for discriminating cryptic and/or very closely related species and reconstruction of phylogenetic relationships within various speciose nematode groups, including members of this genus (Pieterse et al., 2017; Zhang and Liu, 2020; Mwamula et al., 2024). Linking molecular DNA barcodes to morphological data is ideal to improve, if not solve, the taxonomic status of morphologically unresolved species (or genera) within nematode groups. However, Pellioditis species have been molecularly characterized using primarily ribosomal DNA fragments, especially the commonly used 18S-rRNA and 28S-rRNA genes. Unfortunately, the two genes appear to be less informative when comparing cryptic or closely related species of this genus. For instance, P. californica and P. papillosa display a sequence divergence of only 0.7% and 0.8% in 18S-rRNA and 28S-rRNA genes, respectively (Tandingan De Ley et al., 2016). In the current study, the partial 28S-rRNA gene is almost identical to that of P. zhejiangensis and unidentified Pellioditis sp. despite the significant differences in 18S-rRNA and ITS-rRNA gene sequences.
The 18S-rRNA and 28S-rRNA genes appear to be highly conserved within closely related species of the genus. The conservativeness of the 18S-rRNA gene in nematode phylogeny is widely known and has been widely discussed (Blaxter et al., 1998; Holterman et al., 2009; van Megen et al., 2009; Mwamula et al., 2024), and the gene is mostly useful for reconstructing deep nematode phylogeny at higher taxonomic levels. As demonstrated by other studies (Powers et al., 2010; Palomares-Rius et al., 2017; Subbotin et al., 2018; Nguyen et al., 2019), the COI gene is a powerful DNA barcoding marker for nematode identification, especially in discriminating between cryptic species despite the recorded evidence for heteroplasmy, introgression, and recombination events in some nematode groups (Subbotin et al., 2020). For Pellioditis species, COI gene sequences have only been reported in a limited number of species, including P. bonaquaense and another unidentified population. The COI gene should therefore be recommended to be used as a complementary gene in the identification process of the species of this genus, as this will supplement the current generic compendia.
Inoculation of the recovered P. koreana n. sp. dauer juveniles onto S. exigua larvae confirmed the entomo-parasitic relationship between the new species and other insect pests. The new species effectively parasitized S. exigua larvae. P. koreana n. sp. parasitized 100% of first instar larvae of S. exigua after 24 h and 48 h at 100 and 150 dauer juveniles per larva. In the second instar larvae, the mortality reached 100% with 150 dauer juveniles per larva after 72 h. No mortality was observed in the control test. P. koreana n. sp. is thus a potential biocontrol agent for this commonly known polyphagous agricultural pest insect.