Marigold (Tagetes erecta L.) belongs to the Asteraceae family and is a herbaceous plant with aromatic, pinnately divided leaves. Commonly known as the African marigold, it is a significant ornamental plant widely cultivated for its vibrant flowers and diverse applications. It is particularly popular in India due to its adaptability to various soil and climatic conditions, ease of cultivation and profuse flowering habit, making it a favourite among flower growers for decorative and religious purposes (Dole and Wilkins, 1999; Chaupoo and Kumar, 2020). The carotenoid content in the petals of the plant is responsible for its diverse spectrum of flower colours, which include yellow, orange and variations of these hues. Studies have shown that the biosynthesis and degradation of carotenoids play a crucial role in determining the flower colour, with specific genes regulating these processes (Zhang et al., 2020).
In addition to its ornamental value, marigold has been studied for its phytochemical and medicinal properties. It exhibits antinociceptive and anti-inflammatory activities, which have been demonstrated through various experimental models (Sharma and Yadav, 2022). The plant also holds potential for sustainable agricultural practices, such as the use of sewage sludge to enhance growth and yield, while also serving as a phytoremediator for heavy metal-contaminated soils (Madanan et al., 2021; Al-Huqail et al., 2023; Khilji et al., 2024). Furthermore, marigold flowers are a source of valuable bioactive compounds like lutein and polyphenols, which can be efficiently extracted using advanced methods like enzyme-assisted extraction (Fu et al., 2019). Overall, marigold is a versatile plant with significant economic, ornamental and medicinal value, making it a subject of interest for further research and development in various fields.
One method to improve plant growth and flowering is the application of biostimulants (BSs). Many of the effects of these products are based on their ability to influence the hormonal activity of plants. BSs can increase yield and quality as well as mitigate environmental stresses (Conrath, 2011; Savvides et al., 2016). BSs are defined as natural or synthetic substances that enhance plant health, growth and stress resistance through various mechanisms. Among the widely studied BSs, Spirulina platensis, Moringa oleifera extracts, palm pollen grains (PPG) and chitosan (CHI) have demonstrated significant potential in improving the growth and flowering of ornamental plants (Kisvarga et al., 2022).
S. platensis, a blue-green microalga, is a potent BS due to its high content of proteins, polysaccharides, pigments (phycocyanin), vitamins and antioxidants. The presence of carotenoids and polyphenols in Spirulina (SPL) provides protection against oxidative damage while stimulating chlorophyll biosynthesis (Ronga et al., 2019). S. platensis is now commercially produced for applications as a food ingredient, animal feed and biofertiliser, owing to its substantial nutritional profile (Sanchez et al., 2003). Additionally, it has been utilised as a biofertiliser on the sugar beet crop through various application strategies, either as a standalone treatment or in combination with other organic fertilisers (Aly et al., 2008). Moreover, algal-derived BSs have been effectively employed to enhance several plant attributes, including colour intensity, growth rate and flowering, ultimately improving overall crop quality (Kumar et al., 2022). Such algal-derived BSs are Rosmarinus officinalis, Lupinus luteus and Capparis cartilaginea (Abd El-Sadek and Ahmed, 2022; Shedeed et al., 2022; Gharib and Ahmed, 2023).
M. oleifera Lam. is widely recognised as a potent BS, with its leaves and flowers serving as vegetables in regions including Arabia, Africa, India and the United States (Siddhurajue and Becker, 2003; Yasmeen et al., 2013). Moringa leaves extracts (MLE) are abundant in natural antioxidants, which have been demonstrated to enhance growth and yield attributes in various crops such as cowpea, lettuce, sunflowers and stevia, while also helping to mitigate environmental stresses (Howladar, 2014; Maishanu et al., 2017; Iqbal et al., 2020; Sardar et al., 2021; Farooq et al., 2023). MLE have also shown enhanced growth and yield attributes in bean, okra and squash, while also helping to mitigate environmental stresses (Howladar, 2014; Abd El-Mageed et al., 2017; Ali et al., 2024). It is a well-known plant rich in bioactive compounds, including zeatin (a cytokinin), vitamins, minerals and antioxidants. The high cytokinin content in MLE enhances cell division and chlorophyll synthesis, maintaining photosynthetic efficiency in plants especially subjected to drought and salinity stress (Nikkon et al., 2003; Arif et al., 2023). Foliar application of Moringa extracts has been shown to improve the uptake of essential nutrients like nitrogen, phosphorus and potassium, which are crucial for plant resilience (Mashamaite et al., 2022). Moringa extract applications have increased flower production and stress tolerance by enhancing endogenous phytohormones and reducing oxidative damage (Di Sario et al., 2025).
CHI is an oligosaccharide BS obtained by heating chitin and subsequently removing calcium and proteins. It acts as a plant defence elicitor, enhancing drought resistance and oxidative stress management (Sharp, 2013; Palacio-MáRquez et al., 2022). As a natural, biodegradable and non-toxic polymer, CHI is considered to be a potent metabolic elicitor (Yin et al., 2012). Its use in agriculture to increase plant yield is based on its influence on the molecular biology and biochemistry of plant cells. CHI has demonstrated effectiveness in stimulating growth across the Ricinus communis plant (Sara et al., 2012), further supporting its role in enhancing plant productivity (Farouk et al., 2011; Salachna and Zawadzińska, 2014) and has been shown to boost photosynthesis and chlorophyll content. CHI spraying increased the photosynthetic rate and chlorophyll content in Pinellia ternate and Brassica juncea (Dzung and Thang, 2004; Chen et al., 2023; Ningsih and Sari, 2023).
PPG represent the male reproductive cells of flowers, produced by most plant species (Hassan, 2011). As BSs, PPG derived from Phoenix dactylifera are rich in sugars, proteins, minerals, vitamins, carotenoids, enzymes, phytohormones, amino acids, fatty acids and antioxidants (Hassan, 2011; Bishr and Desoukey, 2012; Basuny et al., 2013; Farouk et al., 2015). After extraction, these bioactive compounds can be applied to plants to enhance stress tolerance. Notably, the high levels of auxins and gibberellins in PPG promote robust root and shoot development, thereby improving drought resistance in plants (Hassan, 2011). The presence of flavonoids and phenolic compounds in PPG enhances oxidative stress protection by scavenging reactive oxygen species (ROS) generated under stress conditions (Nadeem et al., 2016). Studies have shown that PPG foliar application enhances flowering and biomass production in Ocimum basilicum under drought stress (Taha et al., 2020).
BSs play a crucial role in the sustainable production of marigold by enhancing growth, flowering and yield while reducing the need for chemical inputs (Soppelsa et al., 2019; Fatima et al., 2021; Rakkammal et al., 2023). This makes them an essential component in ecological and organic farming practices, supporting both plant health and environmental sustainability. Therefore, the objective of this study was to assess the responses of T. erecta plants to BS foliar spray (SPL, Moringa leaves, CHI and PPG) extracts on the vegetative growth and flowering under a newly studied region.
Two field experiments were carried out at Kafr El Battikh district, Damietta Governorate, Egypt during the 2022 and 2023 summer seasons to study the effect of foliar application of SPL, Moringa leaves, CHI and PPG extracts on marigold (T. erecta).
Seeds of marigold F1 cultivar (‘Antigua Orange’ from Syngenta flowers company) were sown in a plastic greenhouse in foam trays (209 eyes), using a mixture of peatmoss (Shalimar Sphagnum Peat Moss Black—300 L, 100% Organic, Eco-friendly soil conditioner), made from Sphagnum Peat, cocopith and decomposed organic nutrients added with humic and seaweed extracts. The soil had high moisture holding capacity, hygroscopic property and inter-gain air space, with sand on a 1:1 (v/v) ratio; planting was done on 1 April in the two seasons. Transplants were planted in the open field on 20 May in the two seasons at the 4–6 leaf stage. The plot area was 162 m2 consisting of 15 rows, with 60 cm in between. Each row was 18 m long at 30 cm between each plant. The method used was the drip irrigation system.
The experiment was designed as a Randomised Complete Block Design with three replications (60 plant · rep−1) as follows: Five foliar applications, that is, control (tap water), SPL (0.1 g · L−1), MLE 3%, CHI (0.1 g · L−1) and pollen grains date palm extract (PGPE 0.1 g · L−1). Marigold plants were sprayed with BSs three times during the growing period after 30, 40 and 50 days from transplanting. In both seasons, all cultural practices, that is, weed and pest control were done according to the recommendations of the Egyptian Ministry of Agriculture.
To prepare a 100 mg · L−1 SPL liquid extract, approximately 0.1 g of fine dry SPL powder was added to 1000 mL of distilled water. The mixture was then heated at 50°C with constant stirring for 60 min, allowed to cool, filtered and stored at 4°C until use (Hamouda et al., 2022).
Moringa aqueous extract was prepared by blending 30 g · L−1 of young, fresh Moringa leaves in tap water until a homogeneous suspension was obtained. The mixture was then filtered and diluted with tap water to a final volume of 1 L (Nouman et al., 2012). The characteristics of M. oleifera extract are presented in Table 1.
The quantitative phytochemical result, proximate composition and mineral composition of the aqueous fresh leaves extract of Moringa oleifera (Nweze and Nwafor, 2014).
| Mineral composition | Value (g · 100 g−1) | Nutrient composition | Value (g · 100 g−1) | Phytochemical extract | Value (g · 100 g−1) |
|---|---|---|---|---|---|
| Nitrogen | 3.03 | Carbohydrate | 57.01 | Anthraquinone | 11.68 |
| Calcium | 2.09 | Protein | 18.92 | Tannins | 9.36 |
| Potassium | 1.62 | Fibre | 9.31 | Terpenoids | 4.84 |
| Sulphur | 0.85 | Ash | 7.95 | Flavonoid | 3.56 |
| Magnesium | 0.48 | Moisture | 4.09 | Steroids | 3.21 |
| Phosphorous | 0.44 | Fats | 2.74 | Alkaloids | 3.07 |
| Iron | 0.03 | Saponins | 1.46 | ||
| Copper | 0.01 | Carotenoids | 1.16 | ||
| Zinc | 0.005 | Cardiac glycoside | 0.36 | ||
| Anthocyanin | 0.06 |
Due to its limited solubility in water, the required amount of CHI was first dissolved in 100 mL of distilled water with acetic acid added to achieve a concentration of 100 mg · L−1. This solution was then diluted to a total volume of 3000 mL. The CHI solution was manually applied by a spray pump (Sheng et al., 2022).
Pollen grains were collected at the end of March from Egyptian date palms (P. dactylifera) in Damietta, Egypt. The extraction of the PPG was carried out as per the method of Taha et al. (2020). The crude extract was analysed, and its chemical constituents, expressed on a dry weight basis, are presented in Table 2.
Chemical constituents of palm pollen grains extract (PPGE) (Taha et al., 2020).
| Component | Unit | Value | Component | Unit | Value |
|---|---|---|---|---|---|
| Moisture | g · 100 g−1 | 28.90 ± 1.64 | Zinc (Zn) | g · kg−1 | 2.72 ± 0.12 |
| Ash | 6.20 ± 0.42 | Copper (Cu) | 3.03 ± 0.16 | ||
| Protein | 31.00 ± 1.52 | Sodium (Na) | 0.52 ± 0.02 | ||
| Total free amino acids | 30.20 ± 1.16 | Soluble phenols | 0.72 ± 0.03 | ||
| Free proline | 0.34 ± 0.01 | Total flavonoids | 0.61 ± 0.02 | ||
| Soluble sugars | g · kg−1 | 14.20 ± 0.31 | Total carotenoids | 14.20 ± 0.24 | |
| Phosphorus (P) | 9.04 ± 0.54 | Vitamin C (ascorbic acid) | 1.06 ± 0.01 | ||
| Calcium (Ca) | 2.49 ± 0.13 | Vitamin A | IU · kg−1 | 747 ± 12.40 | |
| Magnesium (Mg) | 3.42 ± 0.18 | Vitamin E | 335 ± 5.42 | ||
| Potassium (K) | 8.63 ± 0.49 | DPPH (antioxidant activity) | % | ||
| Sulphur (S) | 6.16 ± 0.32 | Phytohormones | |||
| Molybdenum (Mo) | 2.94 ± 0.16 | Indole-3-acetic acid | mg · kg−1 | 85.20 ± 1.38 | |
| Boron (B) | 2.98 ± 0.14 | Gibberellins | 4.92 ± 0.03 | ||
| Iron (Fe) | 4.55 ± 0.20 | Cytokinins | 6.74 ± 0.05 | ||
| Manganese (Mn) | 2.92 ± 0.17 |
Values are means (n = 5) ± SE.
DPPH, 2,2-diphenyl-1-picrylhydrazyl; SE, standard error.
Plant height was measured in centimetres from the soil surface to the highest point of the plants for each treatment before harvesting at the end of July. The number of main branches was determined by counting the main branches from each plant at the end of July. Plant fresh weight was measured in grams after harvesting on 1 August. Plant dry weight (g) was taken as follows: the plants were placed in an envelope and dried naturally in the open, and then dried in a hot air dehydrator oven at 70°C till constant weight was reached.
Flowering attributes, that is, diameter of flowers (cm), were calculated by randomly picking 10 physiologically mature flowers from 10 plants per replicate and per treatment, fresh weight of flower (g), number of flowers per plant (No. of flowers · plant−1), flowers yield per plant (g) and flowers yield per hectare (t · ha−1), were measured at full flower maturity determined by visual observation 60 days after transplanting in an open field, after three sprays. Harvesting was done three times at full opening stage starting from 10 to 30 July for every 10 days for the two seasons. Flower diameter was measured by vernier callipers, whereas flower weight was measured with a digital scale (SF-400). Also determined were the number of days to the first flower bud initiation (NDFBI), number of days to opening the first flower (NDOFF), duration of flowering (DF) and flowering rate% (FR). The FR was calculated as the percentage of plants that reached full bloom relative to the total number of plants in the plot at a specific observation date and the flowering index after 10 days from bud initiation was measured by recording the total number of buds, half-opened flowers and fully opened flowers per plant 10 days after the appearance of the first flower bud.
Length of roots was measured in centimetres from the soil surface to the tip point of the root for each treatment after harvesting:
Root fresh weights were measured in grams after harvest. For root dry weight (g), the roots mentioned above were placed in an envelope and dried naturally in the open, then oven-dried at 70°C till constant weight was reached.
Soil samples were collected from the experimental site at Damietta University at depths of 0–20, 20–40 and 40–60 cm. The samples were air-dried, ground and passed through a 2-mm sieve, then analysed in the Central Laboratory for Soil, Water and Plant Studies to determine the soil’s physical and chemical properties prior to cultivation (Table 3).
Average values of some physical and chemical soil properties for the experimental site as mean values of the two growing seasons.
| Soil layer depth (cm) | Particle size distribution (%) | Soil-water constant | ||||||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Texture | Bulk density (g · cm−3) | F.C 1 (%, wt/wt) | P.W.P 2 (%, wt/wt) | A.W 3 (%, wt/wt) | |
| 0–20 | 79.17 | 15.62 | 5.21 | Sandy | 6.76 | 3.7 | 1.85 | 1.85 |
| 20–40 | 89.58 | 7.29 | 3.13 | Sandy | 6.89 | 3.2 | 1.60 | 1.60 |
| 40–60 | 83.33 | 12.50 | 4.17 | Sandy | 6.85 | 3.0 | 1.50 | 1.50 |
| Mean | 84.02 | 11.81 | 4.17 | Sandy | 15.93 | 3.3 | 1.65 | 1.65 |
| Chemical soil characteristics | ||||||||
| Soil layer depth (cm) | Soluble cations (mEq · L−1) | Soluble anions (mEq · L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH4 1:2.5 | EC dSm−1 | Ca2+ | Mg2+ | Na+ | K+ | CO32– | HCO3– | Cl– | SO42– | |
| 0–20 | 6.50 | 2432 ppm | 50 ppm | 102 ppm | 155 ppm | 80 ppm | --------- | 70.2 ppm | 81.7 ppm | 160.8 ppm |
| 20–40 | 6.66 | 2176 ppm | 90 ppm | 78 ppm | 120 ppm | 60 ppm | -------- | 73.2 ppm | 113.6 ppm | 112.8 ppm |
| 40–60 | 6.70 | 2432 ppm | 90 ppm | 84 ppm | 140 ppm | 70 ppm | -------- | 67.2 ppm | 92.3 ppm | 136.8 ppm |
| Mean | 6.62 | 2346 ppm | 77 ppm | 88 ppm | 138 ppm | 70 ppm | -------- | 70.2 ppm | 95.9 ppm | 136.8 ppm |
F.C. 1, soil field capacity; P.W.P. 2, permanent wilting point; A.W. 3, available soil water, EC, electrical conductivity.
Data presented in Table 4 show the agro-meteorological parameters during the studied interval. The data included air temperature (T, °C), relative humidity (RH, %), wind speed (WS, m · s−1 at 2 m height), evaporation pan (Ep, mm) and rainfall (RF, mm · month−1).
Some agro-meteorological data for the Damietta region (31° 25′ N Latitude, 31° 49′ E Longitude), during the 2022 and 2023 seasons.
| Months | Temp (C°) | RH (%) | WS (m · s−1) | Ep (mm · day−1) | RF (mm · month−1) | |||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | ||||||
| 2022 | April | 23.32 | 15.43 | 18.93 | 71.34 | 4.10 | 5.50 | 0.00 |
| May | 26.77 | 18.95 | 22.50 | 67.16 | 4.30 | 6.60 | 0.00 | |
| June | 30.27 | 23.17 | 26.35 | 65.58 | 3.22 | 7.85 | 0.00 | |
| July | 31.67 | 24.75 | 27.94 | 64.28 | 4.20 | 8.25 | 0.01 | |
| August | 31.74 | 25.80 | 28.32 | 65.50 | 4.17 | 7.57 | 0.00 | |
| Mean 2022 | 28.75 | 21.62 | 24.81 | 66.77 | 4.00 | 7.15 | 0.00 | |
| 2023 | April | 23.95 | 16.31 | 19.68 | 62.70 | 4.38 | 5.69 | 0.26 |
| May | 26.53 | 19.08 | 22.36 | 64.97 | 4.52 | 6.43 | 0.02 | |
| June | 29.83 | 22.51 | 25.76 | 65.91 | 4.32 | 7.39 | 0.07 | |
| July | 33.34 | 25.58 | 29.04 | 64.06 | 4.20 | 8.46 | 0.00 | |
| August | 31.92 | 25.88 | 28.51 | 65.79 | 4.00 | 7.57 | 0.00 | |
| Mean 2023 | 29.11 | 21.87 | 25.07 | 64.69 | 4.28 | 7.11 | 0.07 | |
RH, relative humidity; WS, wind speed; Ep, evaporation; RF, rainfall.
One way analysis of variance (ANOVA) was conducted using the SPSS Statistical Software Package (v.25) (IBM, Armonk, NY, USA). Comparisons of the main treatment means were made using Tukey’s H.S.D at (p = 0.05) (George and Mallery, 2016). Correlation analysis was carried out to examine the relationship between properties of T. erecta affected by BS foliar application.
Plant height is a common measure of vegetative growth influenced by cell elongation and division. Branch number is closely linked to the plant’s ability to capture light and support reproductive development. Dry weight and fresh weight are important indicators of biomass accumulation and overall plant vigour (Brett and Waldron, 1996; Taiz et al., 2015; Bhatla and Lal, 2023). In this study, treatment of marigold plants with BSs significantly (p ≤ 0.05) increased all assessed growth characteristics compared with the corresponding control in the two seasons, as shown in Table 5. The treatment with CHI (0.1 g · L−1) had the maximum growth attributes in most cases, followed by MLE at 3% and PGPE 0.1 g · L−1 without significant differences between them and then SPL 0.1 g · L−1.
Effect of BSs treatments on vegetative growth parameters of Tagetes erecta during the two seasons. 2022 and 2023
| Treatment | Plant height (cm) | Main branches number | Fresh weight of plant (g · plant−1) | Dry weight of plant (g · plant−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 58.43 ± 1.67 c | 44.23 ±2.11 d | 12.67 ± 0.58 c | 11.33 ± 1.53 b | 174.11 ± 9.71 c | 136.89 ± 11.5 c | 30.10 ± 2.37 b | 28.86 ± 1.66 d |
| SPL | 65.40 ±2.20 be | 58.93 ± 1.70 c | 14.67 ± 0.58 b | 14.67 ±0.58 a | 282.27 ± 12.6 b | 216.61 ± 1.14 b | 50.76 ±2.31 a | 44.66 ± 1.91 be |
| MLE | 69.27 ± 1.66 b | 59.03 ± 1.85 c | 16.67 ±0.58 a | 17.00 ± 1.00 a | 364.79 ± 30.8 a | 275.49 ± 9.6 a | 51.07 ± 1.27 a | 42.21 ± 0.96 c |
| CHI | 77.33 ± 1.76 a | 72.90 ±1.35 a | 16.33 ±0.58 a | 15.67 ±0.58 a | 297.76 ± 12.06 b | 261.20 ± 17.1 a | 54.21 ± 1.38 a | 50.85 ±2.17 a |
| PGPE | 72.47 ± 1.57 b | 66.87 ± 0.23 b | 14.33 ± 0.58 b | 16.67 ±0.58 a | 306.26 ±6.01 b | 214.84 ± 2.6 b | 53.73 ± 1.85 a | 47.07 ± 1.91 ab |
Values are means (M) ± SD of triplicate trails. In the columns M ± SD, having the same superscript letters are not significantly different at the 5% level. Values within the same column and followed by different letters are significantly different according to the Tukey test (p ≤ 0.05). Control (tap water), SPL: 0.1 g · L−1, MLE: 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1.
BSs, biostimulants; CHI, chitosan; MLE, Moringa leaves extract; PGPE, pollen grains date palm extract; SD, standard deviation; SPL, Spirulina.
CHI treatments produced the tallest plants with heights reaching up to 77.33 cm in the first season, whereas the control plants were significantly shorter by 24.44%. The increased plant height under CHI and other treatments might be related to improved nutrient uptake and hormonal balance, as CHI is known to induce defence responses and stimulate growth-related pathways and could also replace synthetic cytokinin, which could advance sustainable agriculture (El Hadrami et al., 2010; Acemi et al., 2018; Kumaraswamy et al., 2018). The results also showed that MLE treatment had the highest number of main branches (17.00 and 16.67) in the two seasons, respectively, with significant (p ≤ 0.05) differences over the control, which had considerably fewer branches. Enhanced branching under BS treatments could be attributed to the presence of growth regulators (such as cytokinins and auxins) that are often found in MLE (Anwar et al., 2007; Zulfiqar et al., 2020).
The dry weight data showed that treatments such as CHI and MLE generally resulted in higher biomass accumulation compared with the control. For example, in the first season, CHI recorded the highest significant dry weight (54.21 g · plant−1). Fresh weight followed a similar trend, with treatments like MLE and CHI producing higher values compared with the control. This positive effect may be due to MLE being rich in natural hormones, vitamins and antioxidants. Its high performance in enhancing fresh weight and branching can be explained by its ability to stimulate cell division and elongation (Anwar et al., 2007).
The bioactive compounds in MLE may improve nutrient uptake and assimilation, leading to increased biomass and a greater number of branches. CHI acts as a biopolymer that not only promotes growth but also elicits plant defence responses. Its positive effects on dry weight and plant height suggest that it may enhance both the structural and physiological aspects of growth by improving nutrient absorption and modulating stress responses (El Hadrami et al., 2010; Acemi et al., 2018). SPL also performed well in the first season, though its significance grouping sometimes overlapped with others. These differences suggest that BS may enhance water retention and nutrient assimilation, thereby increasing both the dry and fresh mass of the plants. The application of BSs, especially MLE and CHI, significantly improved the vegetative growth parameters of T. erecta. These improvements are evident in enhanced biomass production (both dry and fresh weight), increased branching and greater plant height. The underlying mechanisms likely involve improved nutrient uptake, enhanced hormone-like activity and induced stress tolerance. These results are in conformity with the other findings (Tavares et al., 2020; Barna et al., 2021; Zeljković et al., 2023) on T. erecta and Tagetes patula, which supports the use of such treatments to optimise plant growth in agricultural practices.
Weight, diameter and number of flowers are important indicators of flower robustness and market quality. Flower size is a critical quality trait that influences both ornamental appeal and potential commercial value. The number of flowers reflects the reproductive capacity and overall productivity of the plant (Mahjoub and Allawi, 2022). The data are presented in Table 6 for two seasons. According to the results, all extracts increased the number of flowers per plant when compared with the control; however, the highest increases were recorded as 55.55%, 49.98%, 46.98% and 42.08% and 63.47%, 56.25%, 54.84% and 52.27% in the two seasons, respectively, for MLE, SPL, PPGE and CHI. By contrast, CHI treatment achieved the highest diameter of flower and weight of flower in the two seasons.
Effect of BSs treatments on the number of flowers per plant, diameter of flower (cm) and weight of flower (g) of Tagetes erecta during the 2022 and 2023 seasons.
| Treatment | Number of flowers per plant | Diameter of flower (cm) | Weight of flower (g) | |||
|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 14.67 ± 2.08 c | 14.00 ± 0.0 c | 5.06 ± 0.76 d | 4.99 ± 0.18 b | 3.38 ± 0.22 d | 5.38 ± 0.85 d |
| SPL | 29.33 ± 0.58 ab | 32.00 ± 2.0 b | 5.64 ± 0.07 c | 5.49 ± 0.21 b | 5.30 ± 0.16 b | 7.31 ± 0.06 b |
| MLE | 33.00 ± 2.00 a | 38.33 ± 0.58 a | 5.65 ± 0.11 c | 5.49 ± 0.29 b | 5.50 ± 0.16 ab | 7.49 ± 0.06 ab |
| CHI | 25.33 ± 1.53 b | 29.33 ± 0.58 b | 6.69 ± 0.10 a | 6.46 ± 0.34 a | 5.82 ± 0.15 a | 7.67 ± 0.09 a |
| PGPE | 27.67 ± 1.53 b | 31.00 ± 1.0 b | 6.30 ± 0.13 b | 6.33 ± 0.25 a | 4.62 ± 0.23 c | 6.60 ± 0.09 c |
Values are means (M) ± SD of triplicate trails. In the columns M ± SD, having the same superscript letters are not significantly different at the 5% level. Values within the same column and followed by different letters are significantly different according to the Tukey test (p ≤ 0.05). Control (tap water), SPL: 0.1 g · L−1, MLE: 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1.
BSs, biostimulants; CHI, chitosan; MLE, Moringa leaves extract; PGPE, pollen grains date palm extract; SD, standard deviation; SPL, Spirulina.
Flower diameter and weight were increased by 24.36% and 22.76% and 31.27% and 29.86% in the two seasons, respectively. This means, MLE (3%) increased the number of flowers at the expense of diameter and weight. Treatments with MLE and SPL tend to show higher values in flower weight and diameter compared with the control.
In terms of the number of flowers per plant, treatments that enhance vegetative growth (Table 5) generally translate into a higher reproductive output. The statistical grouping confirms that these differences are significant (p ≤ 0.05). Enhanced flower weight and size under treatments like MLE and SPL can be attributed to improved nutrient assimilation and hormonal stimulation. Natural bioactive compounds in MLE (e.g. cytokinins and antioxidants) promote both cell division and expansion, resulting in larger and heavier flowers. Similarly, the nutrient-rich profile of SPL supports metabolic activities that favour flower development (Gopalakrishnan et al., 2016; Soni et al., 2021). Increased flower number further suggests that these treatments not only improve individual flower quality but also stimulate overall reproductive efficiency. These results are in conformity with the findings of Russo et al. (1993), Zeljković et al. (2023) and Abdel-Wahed et al. (2024), who reported that the application of BSs like humic acids, amino acids and seaweed extracts improves rose and marigold cultivars, plant height, flower diameter and number of flowers. These substances also promoted earlier flowering and increased the overall biomass of marigold plants tested in the present study.
Figures 1 and 2 show that the application of BSs significantly alters the flowering behavior of T. erecta compared with the control (tap water). Key flowering parameters such as the number of days to first flower bud initiation (NDFBI), days to first flower opening (NDOFF), DF and FR were improved under treatments with (MLE) > (PGPE) > (SPL) > (CHI) in descending order. It is clearly shown that MLE recorded the least number of days for first flower bud initiation (35 days) followed by SPL (37 days), PGPE (38 days) and then CHI (40 days), whereas the maximum number of days (45 days) was recorded with the control. The data suggest that all BS treatments significantly impacted the timing of bud initiation and flower opening, as well as the duration and rate of flowering, compared with the control. This indicates that applying these BSs could potentially enhance the flowering process in marigold by accelerating flower development and possibly increasing the flowering period and rate. The reduction in NDFBI and NDOFF across BS treatments indicates that these substances may be modulating hormonal balances that control flowering time. For instance, MLE is known to be rich in cytokinins and gibberellins, which can promote cell division and early floral induction (Du Jardin, 2015; Bulgari et al., 2019). Similarly, PGPE and SPL contain bioactive compounds and phytohormones, such as gibberellins, which are instrumental in accelerating flowering processes.
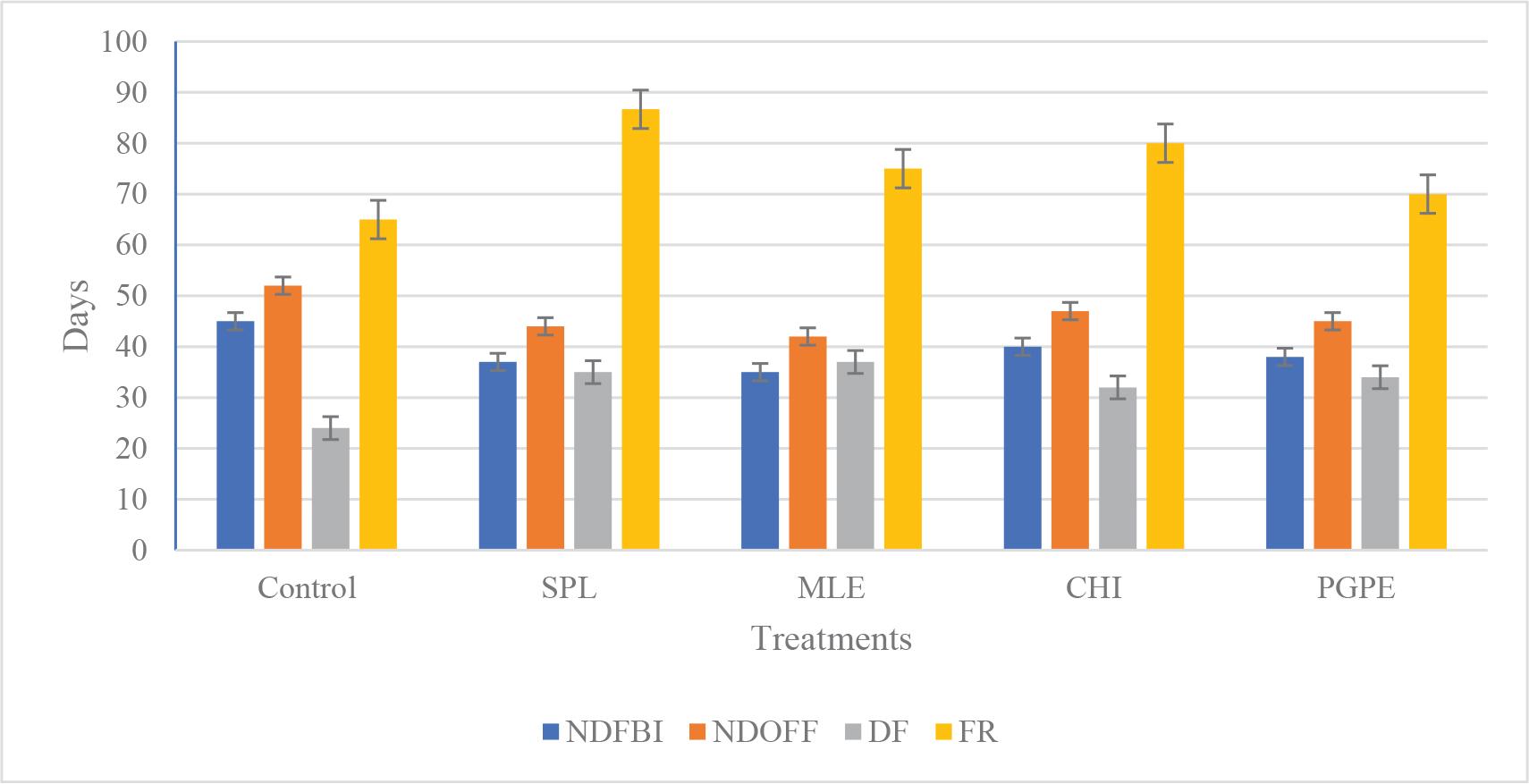
Effect of BSs treatments on flowering behaviours of Tagetes erecta. SPL: 0.1 g · L−1, MLE: 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1. BSs, biostimulants; CHI, chitosan; DF, duration of flowering; FR, flowering rate%; control (Tap water); MLE, Moringa leaves extract; NDFBI, number of days to the first flower bud initiation; NDOFF, number of days for the opening of first flower; PGPE, pollen grains date palm extract; SPL, Spirulina.
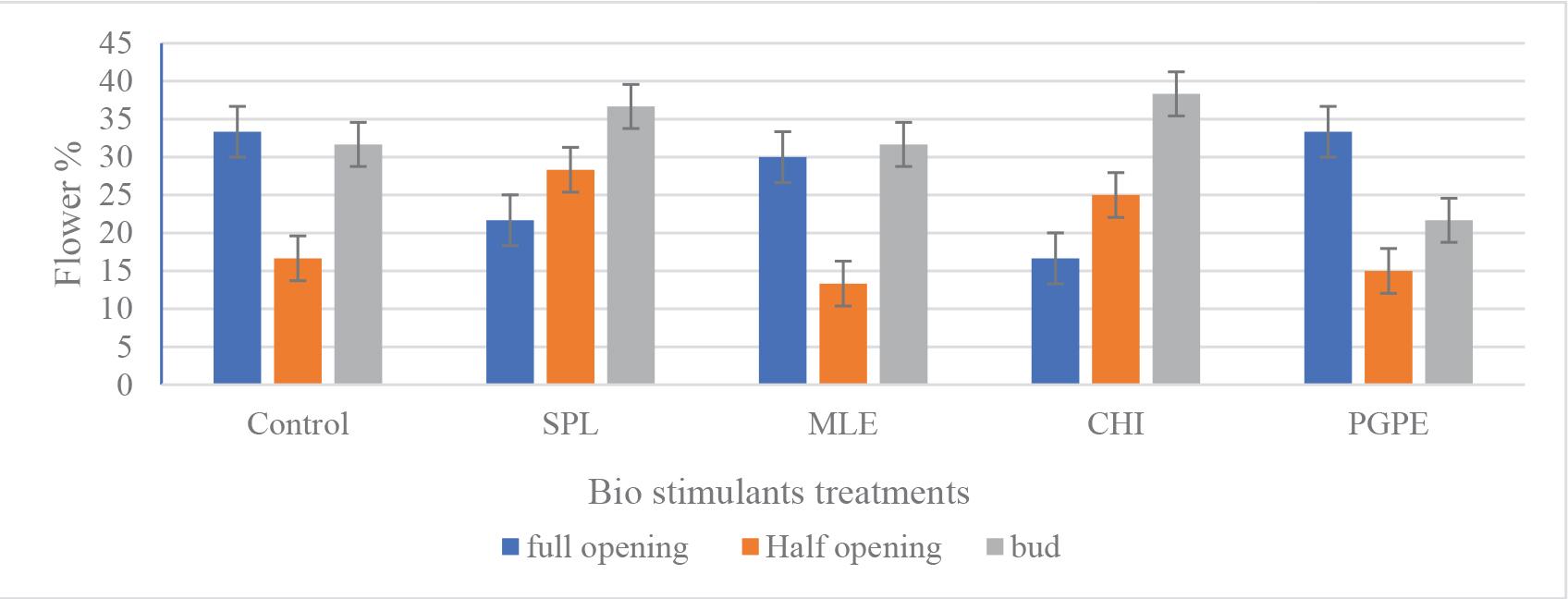
Effect of biostimulant treatments on flowering index of Tagetes erecta. Control (tap water), SPL: 0.1 g · L−1, MLE: 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1. Flower opening stage (full opening; half-opening and bud). CHI, Chitosan; MLE, Moringa leaves extract; PGPE, pollen grains date palm extract; SPL, Spirulina.
An increase in the DF and FR suggests that BS treatments not only initiate flowering sooner but also sustain it longer. This could result from improved nutrient uptake and enhanced metabolic activity, as BSs are reported to boost plant vigour and stress tolerance (Bulgari et al., 2019; Franzoni et al., 2022). The data suggest that treatments with SPL significantly impacted the maximum flower rate% compared with other treatments and the control. This indicates that applying SPL, enhanced nutrient uptake with its array of amino acids, vitamins and minerals, may improve overall plant health, indirectly promoting more robust flowering. CHI is known for eliciting defence responses and could be triggering secondary metabolic pathways that favour prolonged flowering (Hadwiger, 2013). Extended flowering may contribute to better ornamental quality and potentially higher seed or fruit yields in commercial applications. The findings in Figure 2 are in line with the current literature on plant BSs, supporting their role in modulating growth and developmental processes to achieve improved flowering performance. This is consistent with other studies on T. erecta and T. patula, highlighting the beneficial effects of BSs on plant growth and flowering characteristics (Du Jardin, 2015; Thumar et al., 2016; Preeti and Pooja, 2024).
The flowering index is to assign different weights to the various stages of flower development (full opening, half-opening and bud) (Figure 3) and then combine these scores, which is a composite measure that likely integrates several flowering parameters (such as timing, duration and intensity) into a single metric. The integrated data from Tables 5 and 6, along with Figures 2 and 4, provide compelling evidence that BS treatments, particularly those involving CHI and SPL markedly enhance both the qualitative and quantitative aspects of flowering in T. erecta. The observed improvements are evident across several key parameters: enhanced flower quality traits, such as increased flower weight and diameter, and superior reproductive output, reflected in a higher number of flowers per plant and elevated overall yield per hectare. These enhancements are driven by multiple underlying physiological mechanisms, underscoring the effectiveness of these treatments in optimising floral productivity and quality.

Progressive Tagetes erecta and flower development stages at the field experiment.

Tagetes erecta flower at the end of the experiment treated with BSs. BSs, biostimulants; CHI, chitosan; MLE, Moringa leaves extract; SPL, Spirulina.
Figure 2 demonstrates that BS treatments generally lead to an improvement in the flowering index compared with the control. This suggests that the active compounds in these BSs positively affect flower development. For instance, treatments like MLE and CHI may enhance metabolic and hormonal pathways that accelerate or improve the quality of flower opening. This is in line with previous research indicating that natural extracts can stimulate flowering and improve ornamental quality in various species similar to the Chrysanthemum morifolium and Gerbera jamesonii (Kisvarga et al., 2022; Mahjoub and Allawi, 2022). The inclusion of different flower opening stages (bud, half-opening and full opening) highlights that the effect of BS may vary depending on the plant’s developmental stage. Such variations could be due to changes in the plant’s hormonal sensitivity or resource allocation as the flower matures. For example, early-stage treatments might enhance bud development, while later applications could improve full bloom characteristics. These stage-specific responses are supported by findings in the literature where the timing of BS application is critical for maximising benefits which contribute to more vigorous growth and enhanced reproductive performance (El Hadrami et al., 2010; Du Jardin, 2015; Drobek et al., 2019; Sharma and Yadav, 2022). Higher values of the flowering index in BS treated plants indicate not only an accelerated flowering process but also a potentially more robust and sustained flowering performance. This index provides a useful overall assessment of the reproductive vigour imparted by the BS treatments.
The yield data from Table 7 demonstrate that all BS treatments could substantially enhance the production of marigold flowers with significant (p ≤ 0.05) differences, both on a per-plant and per-hectare basis over the control in the two seasons; for instance, MLE (3%) produced significantly higher yields (p ≤ 0.05) compared with the control, with flower yield per plant and per ha reaching 287.01 g and 15.96 t · ha−1 versus 75.35 g and 4.19 t · ha−1 in the control, with a 73.74% increase in the second season, respectively. Similar trends were observed across the two seasons, suggesting that BS not only promoted improved flowering characteristics but also contributed to increased biomass accumulation and overall plant productivity.
Effect of BSs treatments on yield of flower per plant (g) and per ha (t · ha−1), and percentage yield increase of Tagetes erecta during 2022 and 2023 seasons.
| Treatment | Yield of flower per plant (g) | Yield of flower per ha (t · ha−1) | Yield increasing (%) | |||
|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 49.32 ± 3.63 c | 75.37 ± 1.19 d | 2.74 ± 0.2 c | 4.19 ± 0.07 d | – | – |
| SPL | 155.52 ± 7.5 ab | 234.05 ± 15.31 b | 8.64 ± 0.4 ab | 13.30 ± 0.9 b | 68.29 | 67.79 |
| MLE | 181.71 ± 16.13 a | 287.01 ± 6.29 a | 10.10 ± 0.8 a | 15.96 ± 0.3 a | 72.86 | 73.74 |
| CHI | 147.52 ± 8.69 b | 224.90 ± 6.06 bc | 8.20 ± 0.5 b | 12.50 ± 0.3 bc | 66.56 | 66.49 |
| PGPE | 128.05 ± 13.3 b | 204.62 ± 8.04 c | 7.12 ± 0.7 b | 11.38 ± 0.4 c | 61.48 | 63.17 |
Values are means (M) ± SD of three replicates. Within the columns M ± SD, having the same superscript letters are not significantly different at the 5% level. Values within the same column and followed by different letters are significantly different according to the Tukey’s test (p ≤ 0.05). Control (tap water), SPL: 0.1 g · L−1, MLE: 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1.
BSs, biostimulants; CHI, chitosan; MLE, Moringa leaves extract; PGPE, pollen grains date palm extract; SD, standard deviation; SPL, Spirulina.
Treatments with SPL and MLE often displayed higher yield values, while CHI and PGPE showed moderate improvements compared with the control (Figure 4). This statistically significant enhancement in yield highlights the potential of BS to optimise reproductive output, likely through mechanisms such as improved nutrient uptake, particularly nitrogen, phosphorus and potassium, which are crucial for plant growth. They also help plants better withstand temperature stress and other environmental challenges, reducing the need for chemical fertilisers and minimising environmental impact (Gopalakrishnan et al., 2016; Thumar et al., 2016; Drobek et al., 2019; Zeljković et al., 2023).
The observed yield improvements may be linked to several underlying physiological mechanisms, such as MLE and PGPE, which contain phytohormones that can regulate processes associated with flowering and fruiting. Enhanced levels of cytokinins and gibberellins, for example, have been associated with increased cell division and expansion in reproductive tissues (Du Jardin, 2015). SPL is well known for its rich composition of amino acids, vitamins and minerals. This enhanced nutritional profile can stimulate more efficient photosynthesis and carbohydrate allocation, which in turn promotes petunia flower development and yield (Sanchez et al., 2003; Elkinany and Shehata, 2023). CHI, one of the treatments evaluated, has been reported to trigger defence responses that mitigate both biotic and abiotic stress. Reduced stress levels may allow plants to allocate more resources to reproductive development, resulting in improved yield performance (Hadwiger, 2013; Ibrahim et al., 2023).
Likewise for the vegetative growth attributes, the roots of Tagetes eracta plants were also significantly (p ≤ 0.05) affected by BSs application (Table 8). Specifically, root length displayed a highly significant increase after application with PGPE 0.1 g · L−1 without significant differences with CHI (0.1 g · L−1) during the second season compared with the control.
Effect of BSs treatments on root parameters of Tagetes erecta during the two seasons, 2022 and 2023.
| Treatment | Root length (cm) | Roots fresh weight (g · plant−1) | Roots dry weight (g · plant−1) | RRE (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Control | 12.83 ± 0.76 c | 9.00 ± 0.00 d | 12.08 ± 0.57 d | 12.39 ± 0.92 d | 2.42 ± 0.29 b | 3.52 ±0.41 c | - | - |
| SPL | 14.93 ±0.21 b | 14.96 ±0.41 b | 24.52 ± 1.02 b | 19.17 ± 0.92 c | 6.53 ±0.13 a | 4.95 ± 0.36 b | 14.07 | 39.84 |
| MLE | 15.13 ± 0.45 b | 14.76 ± 0.25 be | 16.56 ± 0.85 c | 17.15 ± 0.62 c | 3.62 ± 0.40 b | 5.03 ± 0.73 b | 15.20 | 39.02 |
| CHI | 17.67 ± 0.72 a | 13.67 ± 0.47 c | 15.25 ±0.91 c | 29.61 ± 1.2 a | 6.27 ± 1.04 a | 7.53 ±0.13 a | 27.39 | 34.16 |
| PGPE | 18.50 ±0.78 a | 18.47 ± 0.76 a | 31.55 ±0.79 a | 24.41 ± 1.2 b | 6.35 ±0.73 a | 7.12 ±0.26 a | 30.65 | 51.27 |
Values are means (M) ± SD of three replicates. Within the columns M ± SD, having the same superscript letters are not significantly different at the 5% level. Values within the same column and followed by different letters are significantly different according to the Tukey’s test (p ≤ 0.05). Control (Tap water), SPL: 0.1 g · L−1), MLE: 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1.
BSs, biostimulants; CHI, chitosan; MLE: Moringa leaves extract; PGPE, pollen grains date palm extract; RRE, Relative root elongation; SD, standard deviation; SPL, Spirulina.
Root length is a critical measure for soil exploration. Longer roots are advantageous for water and nutrient absorption, and the statistical differences corroborate the biological impact of BS treatments on root system architecture. The fresh weight data vary among treatments. The PGPE treatment, in the first season, and the CHI treatment in the second one led to the highest values significantly different, as indicated by p ≤ 0.05 in fresh weight, while there is no significant difference between PGPE and CHI in dry weight. The control treatment might have shown a lower root fresh and dry weight compared with the other treatments (e.g. PGPE vs. control). These differences suggest that PGPE and CHI can improve water uptake and storage in the roots, although their peak effects may vary with seasonal conditions. The fresh weight data vary among treatments. The PGPE treatment, in the first season and the CHI treatment in the second one led to the highest values with significant differences, as indicated by p ≤ 0.05. The control treatment might have shown a lower root fresh weight compared with the other treatment (e.g. PGPE vs. control).
These differences suggest that PGPE and CHI can improve water uptake and storage in the roots, although their peak effects may vary with seasonal conditions. The significance of these differences suggests that treatments like CHI not only enhanced carbon allocation to roots but also contributed to improved plant stability and potential stress resilience. In horticultural cultivation, using CHI oligomers rather than polymers could be a more effective and environmentally acceptable substitute for synthetic cytokinins (Acemi et al., 2018). The statistically significant increases in root dry and fresh weights, as well as root length, are in line with the hypothesis that BSs improve nutrient uptake and hormonal regulation. The work of Hadwiger (2013) supports these mechanisms, suggesting that improved root growth enhances overall plant performance and stress tolerance.
Figure 5 provides a clear visual comparison of how different BSs affected the relative root elongation (RRE, %) in marigold plants. Enhanced RRE% is a crucial indicator of improved root system performance, which supports greater water and nutrient uptake. This, in turn, can lead to better plant growth, increased resilience to stress and improved yield potential (Kisvarga et al., 2022). The figure shows that all BS treatments lead to an increase in RRE% relative to the control. While the exact percentage increases are not specified here, the trends suggest that certain treatments, most notably PGPE 0.1 g · L−1, could be more effective in promoting root elongation by 51.27%, followed by SPL 0.1 g · L−1, MLE 3% and CHI 0.1 g · L−1.
These differences underscore the variable efficacy of BSs depending on pollen grains date palm composition and mode of action; especially PGPE contains various bioactive compounds, including growth regulators and micronutrients (Sayed et al., 2018). These results may be attributed to their specific compositions, which are more effective in stimulating the biochemical pathways involved in root development compared with other treatments. Acemi et al. (2018) reported that during the in vitro propagation of Ipomoea purpurea, CHI increased the average number of roots and caused random root induction; however, it also decreased root elongation. When an oligomeric combination is present, the inhibitory effect of CHI on root elongation becomes more evident.
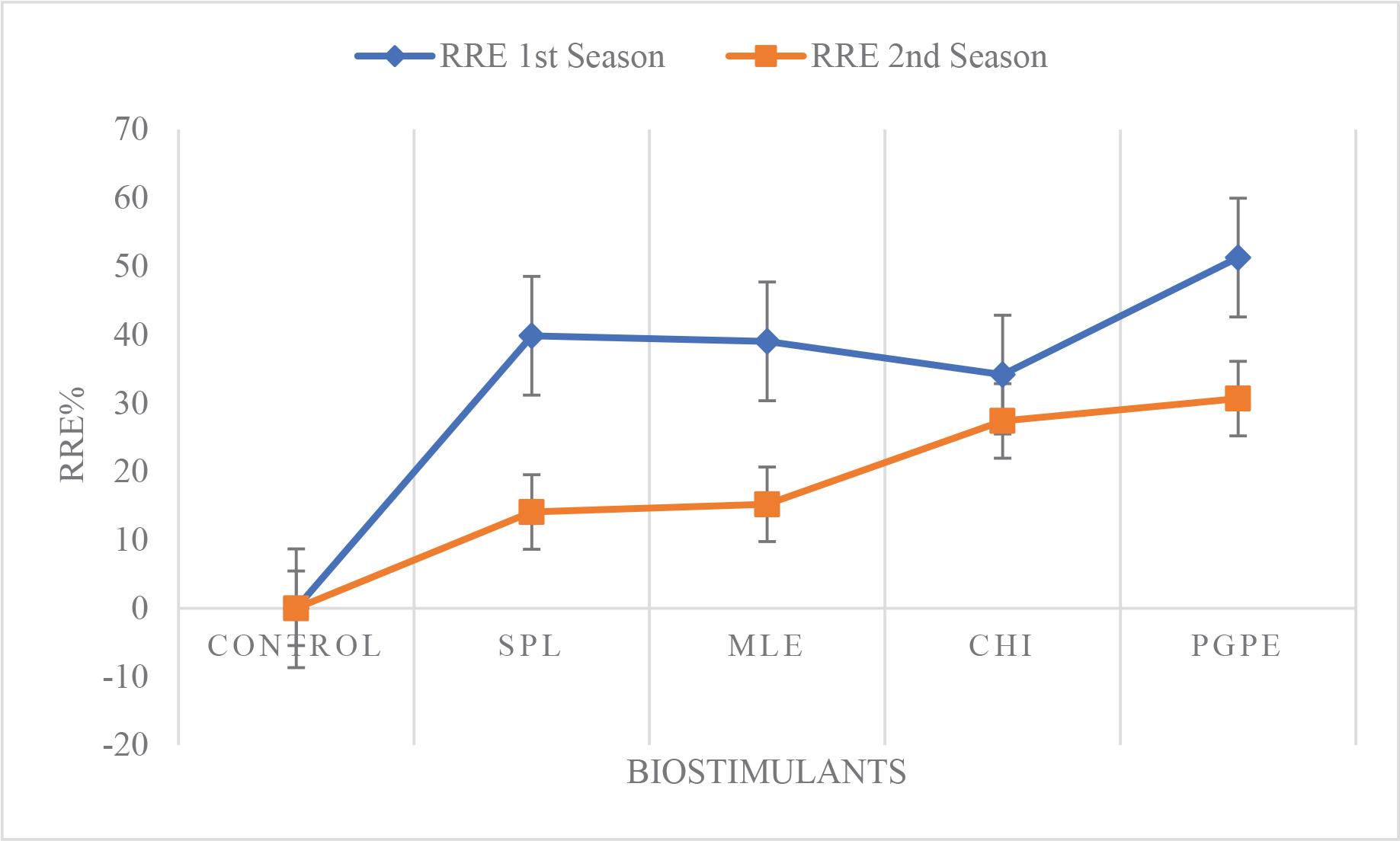
Effect of BSs treatments on RRE, % of Tagetes erecta. Control (tap water), SPL: 0.1 g · L−1, MLE, 3%, CHI: 0.1 g · L−1 and PGPE: 0.1 g · L−1. BSs, biostimulants; CHI, chitosan; MLE, Moringa leaves extract; PGPE, pollen grains date palm extract; RRE, relative root elongation; SPL, Spirulina.
The correlation matrix in Table 9 provides insightful evidence on how BS foliar applications influenced various morphological and yield related attributes of T. erecta. Notably, strong positive correlations existed between vegetative growth parameters such as plant height, number of branches, and both fresh and dry weight and reproductive traits including flower number, diameter and yield per plant and per hectare.
Correlation of properties of Tagetes erecta affected by BSs foliar application.
| Attributes | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Plant height | 1 | ||||||||||
| 2. No. of branches | 0.661** | 1 | |||||||||
| 3. Plant fresh weight | 0.670** | 0.821** | 1 | ||||||||
| 4. Plant dry weight | 0.840** | 0.745** | 0.844** | 1 | |||||||
| 5. No. flower per plant | 0.540** | 0.842** | 0.929** | 0.804** | 1 | ||||||
| 6. Flower diameter | 0.944** | 0.615* | 0.497 | 0.621* | 0.376 | 1 | |||||
| 7. Weight of flower | 0.744** | 0.865** | 0.807** | 0.923** | 0.832** | 0.635* | 1 | ||||
| 8. Flower yield per plant | 0.618* | 0.807** | 0.929** | 0.896** | 0.377 | 0.451 | 0.915** | 1 | |||
| 9. Flower yield per ha | 0.618* | 0.807** | 0.929** | 0.896** | 0.377 | 0.451 | 0.915** | 1.000** | 1 | ||
| 10. Root length | 0.868** | 0.416 | 0.534* | 0.792** | 0.425 | 0.896** | 0.792* | 0.515* | 0.430 | 1 | |
| 11. Root fresh weight | 0.296 | -0.066 | 0.354 | 0.549* | 0.466 | 0.349 | 0.549* | 0.140 | 0.299 | 0.616* | 1 |
| 12. Root dry weight | 0.634* | 0.316 | 0.364 | 0.760** | 0.428 | 0.729** | 0.760** | 0.565* | 0.445 | 0.738** | 0.664** |
BSs, biostimulants.
p ≤ 0.05;
p ≤ 0.01.
Many of the vegetative parameters showed strong, statistically significant positive correlations with reproductive traits. For example, plant height was highly correlated with plant fresh weight and plant dry weight (r = 0.670** and r = 0.840**), suggesting that more vigorous vegetative growth supports greater biomass accumulation. Similarly, the number of flowers per plant had a very high correlation with flower yield per plant (r = 0.944**), indicating that increased flower production directly contributes to higher yields. Strong relationships between flower size and flower weight (with coefficients reaching 0.635*) show that both the number and the quality of the flowers improved with BS treatments.
A larger flower diameter, often associated with higher aesthetic and commercial value, underscores the multifaceted benefits of BS applications in ornamental crop production. The matrix reveals that parameters such as the number of branches, plant fresh weight and plant dry weight are interrelated and collectively contribute to overall plant vigour. While the correlations involving root traits (root length, root fresh weight and root dry weight) are generally moderate compared with the vegetative and reproductive parameters, they still provide valuable insights. For instance, root length showed a moderate positive correlation with plant height (r = 0.868**), which suggests that a well-developed root system may underpin improved above-ground growth. However, the relationships between root fresh weight and yield-related parameters appear less pronounced, reflecting the complexity of how below-ground and above-ground growth interact.
This synergy likely results from improved physiological and metabolic processes induced by BSs, which facilitate better resource allocation towards both growth and reproduction (Hadwiger, 2013). The observed positive correlations align with previous studies that have demonstrated the efficacy of BSs in promoting plant growth through hormonal modulation (e.g. cytokinins and gibberellins in MLE and PGPE), enhanced nutrient assimilation and stress mitigation. These factors collectively improve plant vigour and ultimately yield. For example, enhanced vegetative growth provides a stronger framework for flower development, which is critical in ornamental plants like marigold. In summary, the significant correlations between vegetative and reproductive parameters under BS treatments underscore their potential to improve overall plant performance in T. erecta. The use of significance indicators (** for p < 0.01 and * for p < 0.05) in the table confirms that many of the observed correlations are not due to random chance. These robust statistical associations imply that BS treatments can effectively improve plant performance by simultaneously enhancing multiple growth parameters. This integrated improvement in growth and yield is not only statistically significant but also aligns with the established scientific literature on the benefits of BSs in agriculture (Hadwiger, 2013; Du Jardin, 2015; Bulgari et al., 2019).
The findings of this study demonstrated that BSs, including MLE (3%), CHI (0.1 g · L−1), SPL (0.1 g · L−1) and PGPE (0.1 g · L−1), significantly enhance the flowering performance, yield and overall productivity of T. erecta. These treatments effectively accelerate flowering, increase flower size and number, and improve both ornamental and medicinal value, making them highly promising for commercial horticulture and sustainable crop production. The results indicated a clear ranking of BS efficacy for improving vegetative growth, flowering and yield characteristics: MLE (3%) > CHI (0.1 g · L−1) > SPL (0.1 g · L−1) > PGPE (0.1 g · L−1) in descending order. Notably, PGPE (0.1 g · L−1) was found to be particularly effective in enhancing root characteristics, underscoring its potential for applications targeting root development. By integrating these BS treatments into horticultural practices, growers can achieve more efficient and sustainable production of ornamental and medicinal crops. This approach not only addresses the pressing need for enhanced crop productivity but also aligns with the transition towards environmentally friendly cultivation methods. The study provides robust evidence supporting the use of BSs as a viable strategy to meet the dual objectives of improving yield and promoting sustainability in modern agriculture and horticulture. This version emphasises the practical implications of the findings, aligns with the study’s objectives, and ensures clarity and precision in presenting the outcomes.
Future work might focus on elucidating the precise biochemical pathways involved and optimising application protocols for different environmental conditions. While the article provides clear evidence of the beneficial effects of BSs on flowering behaviour, further research is needed to optimise concentrations, application timing and to elucidate the underlying molecular mechanisms. Such studies could lead to tailored BS formulations for different horticultural species, enhancing sustainable agricultural practices. While the data clearly demonstrate the positive effects of these BSs on the flowering index, further research is warranted to explore the following:
Dose optimisation: identifying the optimal concentrations and application intervals for each BS;
Mechanistic studies: conducting molecular and biochemical studies to elucidate the underlying pathways and gene expressions affected by these treatments;
Long-term effects: assessing the impact of repeated BS applications on overall plant health, productivity and stress resilience over multiple growing seasons.