Figure 1.
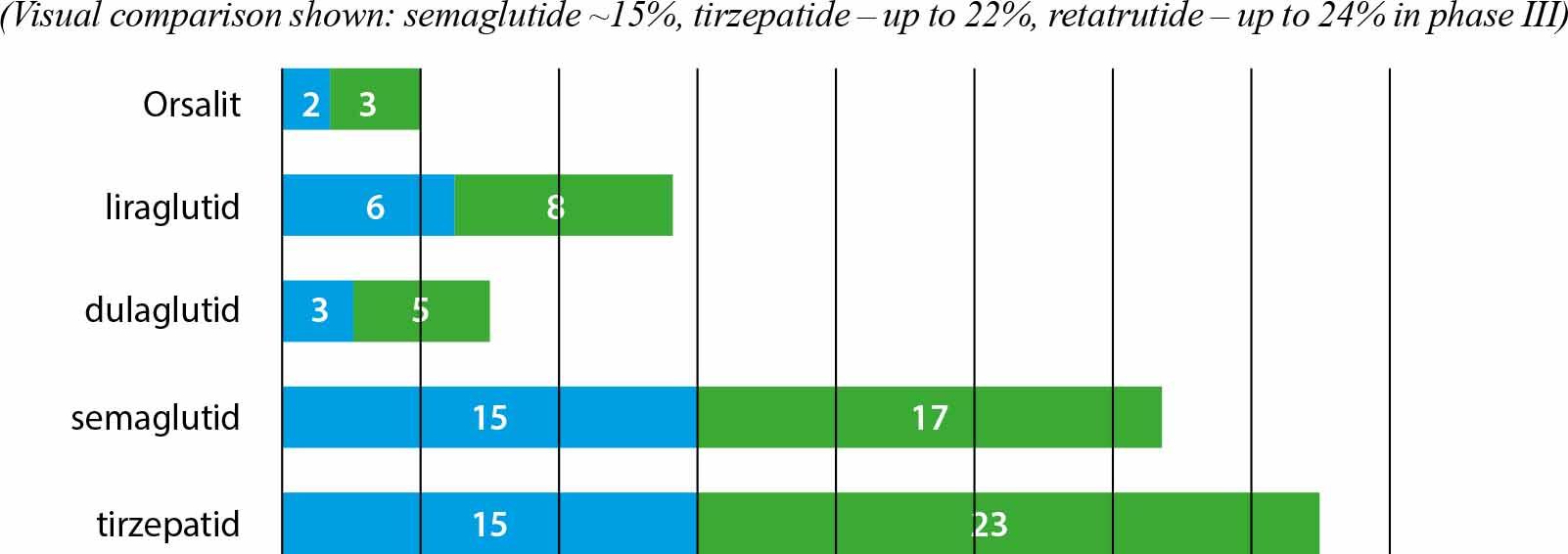
Figure 2.
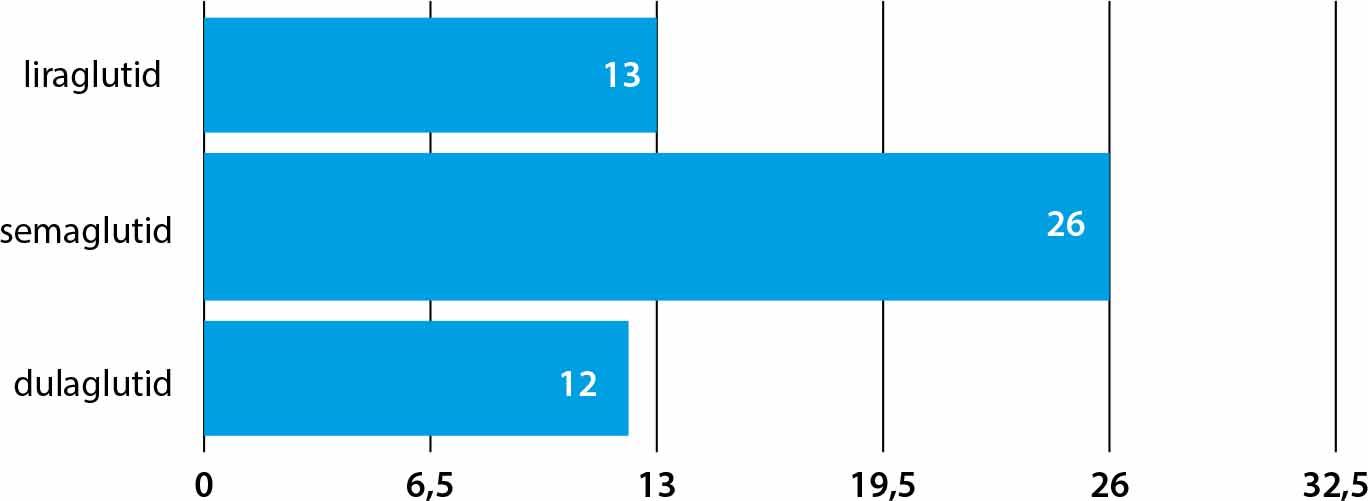
CoMparison of cardiometabolic effects of GLP-1 agonists and GIP/GLP-1 analogues_
| Drug | MACE reduction (%) | HR (vs placebo) | Clinical Trial |
|---|---|---|---|
| Liraglutide | –13% | 0.87 | LEADER |
| Semaglutide | –26% | 0.74 | SUSTAIN-6 |
| Dulaglutide | –12% | 0.88 | REWIND |
| Tirzepatide | ongoing | n/a | SURPASS-CVOT |
Availability of GLP-1 and GIP/GLP-1 drugs in the EU and USA (as of 2025)_
| Drug / Preparation | FDA | EMA | Poland – reimbursement DM2 | Poland – reimbursement obesity | Administration |
|---|---|---|---|---|---|
| Wegovy® (semaglutide) | ✔ (2021) | ✔ (2022) | ✔ | ✗ | s.c. 2.4 mg/week |
| Ozempic® (semaglutide) | ✔ | ✔ | ✔ | off-label | s.c. 0.25–2 mg |
| Rybelsus® (semaglutide) | ✔ | ✔ | ✔ | ✗ | oral |
| Saxenda® (liraglutide) | ✔ (2014) | ✔ (2015) | ✗ | ✗ | s.c. 3 mg/day |
| Mounjaro® (tirzepatide) | ✔ (2022, DM2) | ✔ (DM2) | ✔ | ✗ | s.c. 5–15 mg/week |
| Zepbound® (tirzepatide) | ✔ (2023, obesity) | in procedure (2025) | ✔ (DM2) | ✗ |
Efficacy – summary of results
| Dose (mg) | Body weight reduction (%) | Liver fat reduction (%) |
|---|---|---|
| 8 | –23.8% | –81.4% |
| 12 | –25.9% | –82.4% |
Comparison of the effects of GLP-1 agonists and GIP/GLP-1 analogues in obesity treatment – data from clinical trials_
| Drug | Dose | HbA1c Reduction (%) | Clinical Trials |
|---|---|---|---|
| Orlistat | 120 mg 3×/day | no effect | XENDOS |
| Liraglutide (Saxenda®) | 3 mg/day | 1.0–1.5% | SCALE |
| Semaglutide (Wegovy®) | 2.4 mg/week | 1.5–2.0% | STEP 1 |
| Dulaglutide | 1.5–4.5 mg/week | 1.0–1.5% | AWARD, REWIND |
| Tirzepatide (Zepbound®) | 5–15 mg/week | 2.0–2.4% | SURMOUNT-1, SURMOUNT-5 (2025) |