Incretins are gut hormones secreted in response to food intake. The main ones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Both substances play a key role in the regulation of carbohydrate and energy metabolism.
In individuals with obesity and type 2 diabetes, an impaired incretin response is observed, leading to disturbed secretion of insulin and glucagon, thereby promoting hyperglycemia and excessive appetite [1, 2].
Increases insulin secretion in a glucose-dependent manner.
Inhibits glucagon secretion.
Delays gastric emptying, enhancing satiety.
Acts on the central nervous system, suppressing appetite.
GLP-1 agonists are designed to be resistant to degradation by the enzyme DPP-4, which ensures a longer half-life and clinical efficacy [3].
Stimulates insulin secretion in a glucose-dependent manner.
Influences lipid metabolism and adipogenesis.
In insulin-resistant individuals, its effect is partially impaired.
Although GIP was once considered less relevant in therapy, current studies highlight its potential synergistic role with GLP-1 [4].
Modern therapies, such as tirzepatide, combine GLP-1R and GIPR effects, leading to:
Stronger appetite reduction.
Increased glucose-dependent insulin secretion.
Better glycemic control.
Modulation of visceral fat metabolism.
Preclinical and clinical studies indicate that activation of both receptors produces an additive or synergistic effect on weight reduction and improvement of metabolic parameters [5, 6].
GLP-1 agonists and dual GLP-1/GIP agonists act on the arcuate nucleus of the hypothalamus.
They inhibit NPY/AgRP (orexigenic) neurons.
They activate POMC/CART (anorexigenic) neurons.
The result is reduced appetite and decreased calorie intake.
Neuroimaging (fMRI) has confirmed decreased activity of brain regions associated with reward after treatment with GLP-1 agonists [7].
Pharmacotherapy of obesity has undergone revolutionary changes in recent years. GLP-1 agonists and dual GLP-1/GIP agonists play a key role, offering efficacy comparable to bariatric surgery and proven metabolic benefits.
Liraglutide (Saxenda® 3 mg):
administration: daily subcutaneous injection,
weight reduction: 6–8% [8],
SCALE study: effect stabilization after 56 weeks,
approved for obesity by FDA and EMA.
Semaglutide (Wegovy® 2.4 mg):
administration: once weekly subcutaneous,
weight reduction: 14.9–17% [9],
STEP 1 study (NEJM 2021): –14.9% in 68 weeks,
approved for obesity in the USA and EU (2021–2022).
Dulaglutide (Trulicity®):
administration: weekly,
weight reduction: 3–5% [10],
primarily for DM2, not approved for obesity.
Tirzepatide (Mounjaro®, Zepbound®):
world’s first dual GLP-1/GIP agonist,
administration: once weekly subcutaneous, doses 5–15 mg,
approvals:
- –
FDA 2022–2023 – DM2,
- –
FDA 2023 – Zepbound for obesity and OSA,
- –
EMA – approved for DM2, obesity approval procedure ongoing (2025).
- –
Retatrutide – a novel triple-action obesity therapy
Retatrutide (LY-3437943) is an innovative peptide agonist of three incretin receptors: GLP-1, GIP, and glucagon receptor (GCGR). Its unique triple-action structure provides:
substantial weight reduction (-22–25% within 24–48 weeks),
improvement in glycemia (HbA1c reduction) and metabolic parameters [11],
beneficial impact on liver fat reduction (MASLD/NAFLD) [12].
FDA (USA): not approved, only available in phase III clinical trials (TRIUMPH, SURPASS-CVOT). As of now, FDA does not recognize retatrutide as an approved drug [13, 14].
EMA (EU): not approved; still under global evaluation with no clinical indications approved.
Phase III: central phase III studies planned for 2025–2026, completion will allow later registration submissions [14, 1].
In a phase II study (48 weeks), doses of 8 and 12 mg of retatrutide demonstrated body weight reductions of– 23.8% and – 25.9%, respectively, with liver fat mass reduced by 81–82%.
In phase I/II in patients with type 2 diabetes, improvements in body composition were observed (reduction of fat mass while preserving lean mass), as well as favorable changes in metabolic parameters – glucose, blood pressure, and lipids [15].
Efficacy – summary of results
| Dose (mg) | Body weight reduction (%) | Liver fat reduction (%) |
|---|---|---|
| 8 | –23.8% | –81.4% |
| 12 | –25.9% | –82.4% |
The adverse event profile is consistent with GLP-1/GIP agonists – most commonly nausea, vomiting, and constipation, usually mild and transient. No serious adverse events were reported [15].
Retatrutide represents a new generation of obesity therapies, combining incretin and glucagon activity. Several large phase III trials (e.g., TRIUMPH) are currently ongoing to assess its long-term safety and efficacy.
Preliminary results are very promising, but important considerations remain:
Cost of therapy.
Comparison with tirzepatide.
Impact on comorbidities (e.g., NASH, diabetes).
SCALE (liraglutide):
- –
weight reduction: ~8%,
- –
sustained effect with continued therapy [8],
- –
STEP 1 (semaglutide):
SURMOUNT-1 (tirzepatide):
- –
weight reduction: up to 22.5% at 72 weeks,
- –
HbA1c reduction: 2.0–2.4% [17].
- –
SURMOUNT-5 (2025):
- –
comparison: tirzepatide 10–15 mg vs semaglutide 2.4 mg,
- –
tirzepatide: –20.2% vs semaglutide: –13.7% (P < 0.001) [18].
- –
Comparison of the effects of GLP-1 agonists and GIP/GLP-1 analogues in obesity treatment – data from clinical trials.
| Drug | Dose | HbA1c Reduction (%) | Clinical Trials |
|---|---|---|---|
| Orlistat | 120 mg 3×/day | no effect | XENDOS |
| Liraglutide (Saxenda®) | 3 mg/day | 1.0–1.5% | SCALE |
| Semaglutide (Wegovy®) | 2.4 mg/week | 1.5–2.0% | STEP 1 |
| Dulaglutide | 1.5–4.5 mg/week | 1.0–1.5% | AWARD, REWIND |
| Tirzepatide (Zepbound®) | 5–15 mg/week | 2.0–2.4% | SURMOUNT-1, SURMOUNT-5 (2025) |
Source: Author’s own elaboration based on data from studies: Wilding JPH et al. N Engl J Med. 2021; 384:989–1002. PMID: 33567185; Jastreboff AM et al. N Engl J Med. 2022; 387:205–216. PMID: 35704300; Frias JP et al. Nature Med. 2023; 29:845–854. PMID: 37166354.
GLP-1R:
- –
Glucose-dependent stimulation of insulin.
- –
Glucagon inhibition.
- –
Anorexigenic effect.
- –
GIPR:
- –
Additional insulin stimulation.
- –
Influence on adipocytes.
- –
Synergistic appetite suppression.
- –
Tirzepatide acts on both receptors, producing additive or synergistic effects [6, 18].
HbA1c reduced by 1.0–2.5% in phase III trials.
Particularly beneficial in type 2 diabetes.
FDA and EMA recommend them in patients with DM2 and coexisting obesity [21].
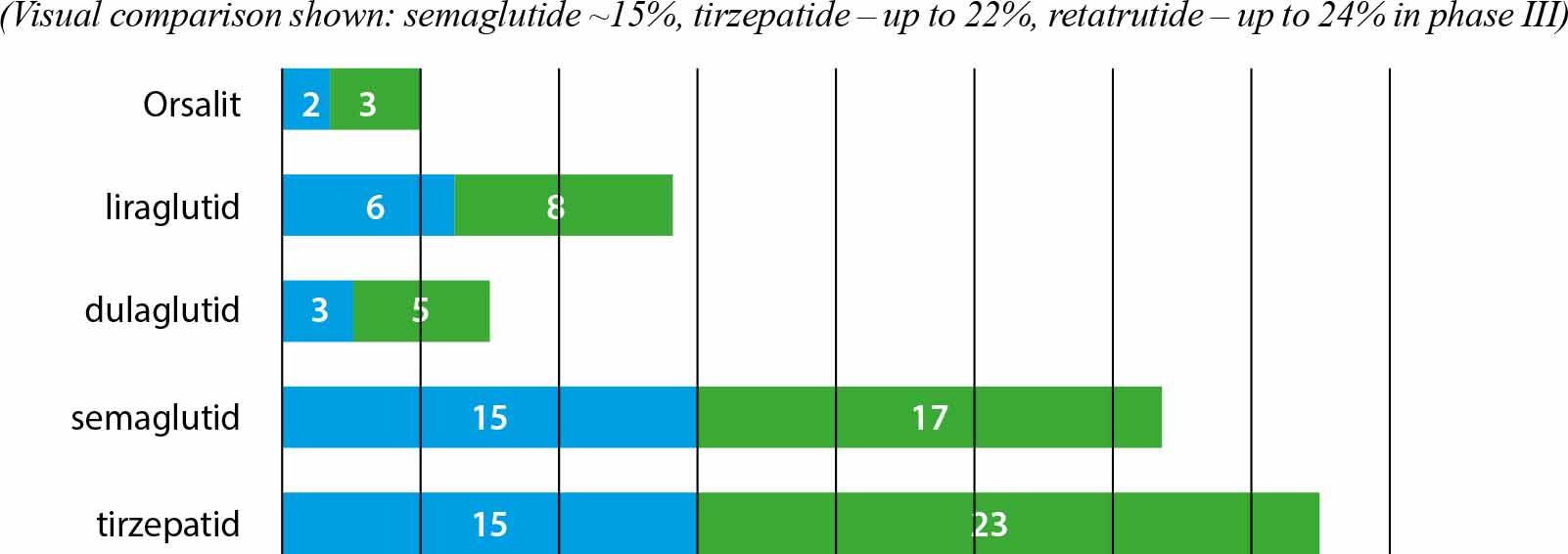
Percentage weight loss in selected studies using GLP-1 agonists and GIP/GLP-1 analogues.
Source: Based on Wilding JPH et al. N Engl J Med. 2021; 384:989–1002. PMID: 33567185; Jastreboff AM et al. N Engl J Med. 2022; 387:205–216. PMID: 35704300; Frias JP et al. Nature Med. 2023; 29:845–854. PMID: 37166354.
The efficacy of GLP-1 agonists and dual GLP-1/GIP agonists in reducing body weight has been confirmed in many phase III randomized clinical trials and meta-analyses up to 2025.
Liraglutide 3 mg (Saxenda®):
- –
average weight reduction: 6–8% [8],
- –
SCALE study: –8% after 56 weeks,
- –
stable effect with continued therapy.
- –
Semaglutide 2.4 mg (Wegovy®):
- –
weight reduction: 14.9–17% at 68 weeks [9],
- –
STEP 1: –14.9% mean vs –2.4% placebo,
- –
effectiveness comparable to bariatric surgery in some post-hoc analyses.
- –
Dulaglutide:
- –
weight reduction: 3–5%,
- –
effect confirmed mainly in patients with DM2 [10].
- –
SURMOUNT-1:
- –
body weight reduction: up to –22.5% at 72 weeks,
- –
placebo-adjusted reduction ~18% [17].
- –
SURMOUNT-5 (2025):
- –
tirzepatide 10–15 mg: –20.2%,
- –
semaglutide 1.7–2.4 mg: –13.7%,
- –
tirzepatide superior to semaglutide by ~6.5% [18].
- –
tirzepatide 10–15 mg > semaglutide 2.4 mg by ~4–5% [19],
stable effect with long-term use,
Appetite reduction (central anorexigenic action).
Delayed gastric emptying.
Improved insulin sensitivity.
Reduction of visceral adipose tissue volume.
Modulation of reward and satiety pathways in CNS [7].
Observational studies (2023–2025) show that discontinuation of therapy leads to partial weight regain (~2/3 within 1–2 years) (21).
Chronic treatment is necessary to maintain effect.
Pharmacotherapy of obesity using GLP-1 agonists and dual GLP-1/GIP agonists has significantly improved treatment options but is also associated with limitations and adverse effects that must be considered in clinical practice.
nausea (20–40% of patients),
vomiting,
diarrhea or constipation,
often transient and subside after several weeks.
dyspepsia,
feeling of fullness.
Studies from 2025 confirm that gradual dose escalation significantly reduces the frequency and severity of these adverse events [19, 20].
Pancreatitis:
- –
rare cases reported in trials and safety reports,
- –
no confirmed increased risk in large meta-analyses.
- –
Medullary thyroid carcinoma (MTC):
- –
theoretical risk based on rodent studies,
- –
no evidence of increased risk in humans (FDA, however, requires a warning label) [20].
- –
CoMparison of cardiometabolic effects of GLP-1 agonists and GIP/GLP-1 analogues.
| Drug | MACE reduction (%) | HR (vs placebo) | Clinical Trial |
|---|---|---|---|
| Liraglutide | –13% | 0.87 | LEADER |
| Semaglutide | –26% | 0.74 | SUSTAIN-6 |
| Dulaglutide | –12% | 0.88 | REWIND |
| Tirzepatide | ongoing | n/a | SURPASS-CVOT |
Source: Author’s own elaboration based on: Marso SP et al. N Engl J Med. 2016; 375:311–322. PMID: 27295427; Gerstein HC et al. Lancet. 2019; 394:121–130. PMID: 31118100; Jastreboff AM et al. N Engl J Med. 2022; 387:205–216. PMID: 35704300; Frias JP et al. Nature Med. 2023; 29:845–854. PMID: 37166354
Cardioprotective mechanisms include reduction in blood pressure (2–5 mmHg), improved lipid profile, and reduced low-grade inflammation.
Large CVOT studies have demonstrated MACE reduction (Figure 2):
Liraglutide (LEADER): –13% (HR 0.87) [16].
Semaglutide (SUSTAIN-6): –26% (HR 0.74) [16].
Dulaglutide (REWIND): –12% (HR 0.88) [15].
Tirzepatide: SURPASS-CVOT results expected in 2025/26.
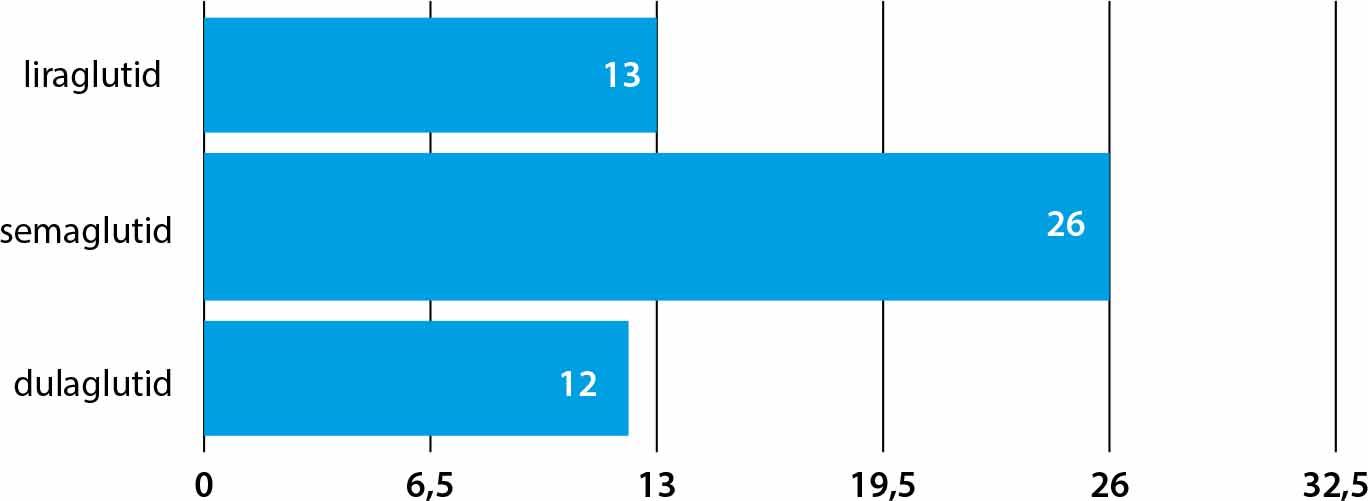
Effects of MACE reduction (%) in clinical trials using modern incretin mimetics
Orlistat was not studied in large randomized CVOTs, thus no data exist on its effect on MACE reduction. Tirzepatide is currently being evaluated in the SURPASS-CVOT study – results expected after 2025. Liraglutide, dulaglutide, and semaglutide have confirmed MACE reduction effects in dedicated clinical trials.
Source: Wilding JPH et al. N Engl J Med. 2021; 384:989–1002. PMID: 33567185; Jastreboff AM et al. N Engl J Med. 2022; 387:205–216. PMID: 35704300; Frias JP et al. Nature Med. 2023; 29:845–854. PMID: 37166354.
Additional effects:
Tirzepatide and semaglutide are significantly more expensive than earlier drugs.
Reimbursement in most countries is limited to DM2.
The weight loss effect disappears after discontinuation.
Observational studies (2023–2025) show regain of ~2/3 of lost weight within 1–2 years without continued treatment [21].
Limited in low- and middle-income countries.
Differences in registrations and reimbursement between FDA and EMA.
The availability and reimbursement of GLP-1 and dual GLP-1/GIP agonist therapies remain key challenges in global obesity treatment. Although these drugs have revolutionized therapeutic approaches, their high cost and limited availability hinder widespread population-level use.
Semaglutide (Wegovy®, Ozempic®, Rybelsus®):
- –
Wegovy® (2.4 mg s.c.) approved by FDA (2021) and EMA (2022) for obesity treatment (BMI ≥30 or ≥27 with comorbidities).
- –
Ozempic® – registered for DM2, used off-label for weight reduction.
- –
Rybelsus® – only oral GLP-1, registered for DM2.
- –
Liraglutide (Saxenda® 3 mg):
- –
approved by FDA (2014) and EMA (2015) for obesity treatment,
- –
daily s.c. injections.
- –
Tirzepatide (Mounjaro®, Zepbound®):
- –
Mounjaro® – FDA and EMA approval for DM2.
- –
Zepbound® (2023) – FDA approval for obesity and obstructive sleep apnea (OSA).
- –
EMA – registration procedure for obesity ongoing in 2025.
- –
USA:
- –
Wegovy® and Zepbound®: reimbursement depends on insurer; manufacturer discount programs available.
- –
Ozempic®, Rybelsus®: reimbursed in DM2.
- –
EU (including Poland):
- –
semaglutide and liraglutide reimbursed only in DM2,
- –
no reimbursement for obesity indication (off-label use in clinical practice),
- –
tirzepatide: reimbursed in DM2, no reimbursement for obesity in 2025.
- –
UK:
- –
NICE in 2023 recommended limited funding of Wegovy® on NHS for selected groups (BMI ≥35 or ≥30 with complications),
- –
reimbursement negotiations for tirzepatide ongoing in 2025.
- –
Availability of GLP-1 and GIP/GLP-1 drugs in the EU and USA (as of 2025).
| Drug / Preparation | FDA | EMA | Poland – reimbursement DM2 | Poland – reimbursement obesity | Administration |
|---|---|---|---|---|---|
| Wegovy® (semaglutide) | ✔ (2021) | ✔ (2022) | ✔ | ✗ | s.c. 2.4 mg/week |
| Ozempic® (semaglutide) | ✔ | ✔ | ✔ | off-label | s.c. 0.25–2 mg |
| Rybelsus® (semaglutide) | ✔ | ✔ | ✔ | ✗ | oral |
| Saxenda® (liraglutide) | ✔ (2014) | ✔ (2015) | ✗ | ✗ | s.c. 3 mg/day |
| Mounjaro® (tirzepatide) | ✔ (2022, DM2) | ✔ (DM2) | ✔ | ✗ | s.c. 5–15 mg/week |
| Zepbound® (tirzepatide) | ✔ (2023, obesity) | in procedure (2025) | ✔ (DM2) | ✗ |
Source: EMA, FDA, ClinicalTrials.gov, registration publications of manufacturers: Novo Nordisk, Eli Lilly (2021–2025).
High cost:
- –
Annual cost of Wegovy®, Zepbound® >10,000 USD in USA.
- –
Limited availability in public systems.
- –
Inequalities in access:
- –
Low- and middle-income countries practically without access to modern therapies.
- –
Reimbursement barriers even within EU.
- –
Systemic challenges:
- –
Need for medical staff education.
- –
Therapy outcome monitoring
- –
Implementation of public health strategies.
- –
Obesity is a chronic metabolic disease of global scope and growing epidemiological significance. In 2025, the number of obese individuals worldwide exceeded 1 billion, posing a serious public health, economic, and social challenge.
The pathophysiology of obesity is based on complex mechanisms: chronic low-grade inflammation, insulin resistance, impaired incretin secretion, and dysregulation of the central nervous system. Numerous comorbidities – type 2 diabetes, hypertension, dyslipidemia, NAFLD, OSA, and cardiovascular diseases – exacerbate the problem and increase mortality.
Incretin pharmacotherapy (GLP-1 agonists and dual GLP-1/GIP agonists) represents the greatest breakthrough in obesity treatment in decades. These drugs allow for body weight reduction of 6–22.5%, improve glycemic control (HbA1c –1–2.5%), decrease visceral adipose tissue volume, and in some cases reduce cardiovascular risk (MACE reduction up to 26% for semaglutide in SUSTAIN-6).
The dual GLP-1/GIP agonist tirzepatide achieves efficacy comparable to bariatric surgery, surpassing semaglutide by 4–6.5% in body weight reduction in phase III trials (including SURMOUNT-5 in 2025). Cardiovascular outcome trial results for tirzepatide (SURPASS-CVOT) are expected in 2025/26 and may confirm additional cardioprotective benefits.
Treatment limitations include high costs, lack of widespread reimbursement for obesity (outside of DM2), need for chronic use, and adverse effects – mainly gastrointestinal. The weight loss effect partially disappears after discontinuation, requiring long-term strategies and patient support.
In summary, incretin therapies represent the foundation of modern obesity treatment. However, their effective implementation requires systemic strategies, reimbursement, patient and medical staff education, as well as an integrated approach including behavioral, dietary, and – in selected patients – bariatric surgical interventions.
Obesity is one of the most serious health challenges of the 21st century, being a chronic disease with multifactorial etiology and numerous metabolic and cardiovascular consequences. The prevalence of obesity is increasing worldwide, including among children and adolescents.
The key pathophysiological mechanism of obesity is insulin resistance, which leads to the development of type 2 diabetes, atherogenic dyslipidemia, hypertension, and non-alcoholic fatty liver disease. Obesity is also associated with obstructive sleep apnea and increased cardiovascular risk.
In recent years, GLP-1 analogues and dual GLP-1/GIP agonists have gained enormous significance in pharmacological therapy. Their mechanism of action includes stimulation of insulin secretion, inhibition of glucagon, delayed gastric emptying, and influence on central satiety mechanisms. Phase III trials have shown that GLP-1 agonists can reduce body weight by 6–17%, and the dual agonist tirzepatide by up to 22.5%, with effects comparable to bariatric surgery.
These drugs additionally demonstrate beneficial metabolic effects (HbA1c reduction by 1–2.5%) and, in some cases, cardioprotective benefits (MACE reduction up to 26% for semaglutide). However, their use requires chronic treatment, is associated with high cost, and carries a risk of adverse events such as nausea or diarrhea.
In conclusion, GLP-1 and GLP-1/GIP analogues represent a breakthrough in obesity treatment, but their implementation requires appropriate integration, patient education, and reimbursement/systemic strategies.