Fig 1.
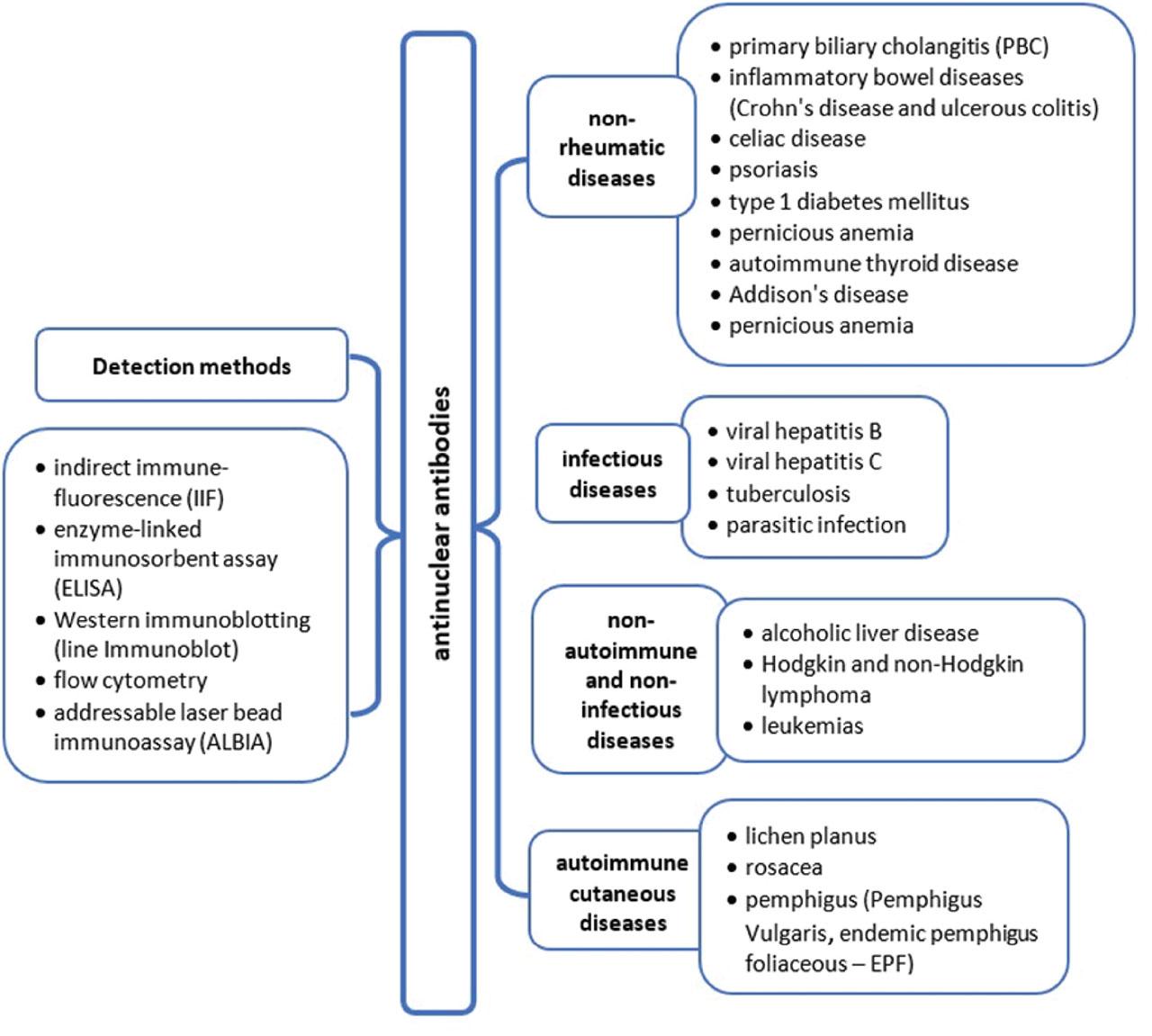
Methods used for ANA detection
| Methods for ANA measurement | |||
|---|---|---|---|
| Method | Method description | Advantages | Limitations |
| IIF |
|
|
|
| ELISA |
|
|
|
| Western immunoblotting (line ImmunoBlot) |
|
|
|
| Flow cytometry |
|
|
|
| ALBIA |
|
|
|
Characteristics of ANAs antibodies in infectious and non-infectious diseases
| ANAs antibodies in infectious diseases | ||||
|---|---|---|---|---|
| Disease | Number of patients | Number of ANAs (+) patients/clinical characteristics | Method of ANAs measurement | Reference |
| Hepatitis C |
|
| IIF | de Castro et al. (2022) |
| N = 48 |
| IIF | Pisetsky (2011) | |
| TB |
|
| ELISA | Shen et al. (2013) |
| n = 1 (case report) |
| IIF | Win et al. (2003) | |
| Parasitic infection | n = 613 |
| ELISA | Mutapi et al. (2011) |
| n = 125 |
| IFAT | Wang et al. (2018) | |
| ANA in non-infectious diseases | ||||
| Diseases | Number of patients | ANA-characteristics | Method | Reference |
| ALD | n = 90 | 63.8% (44 out of 69) | IIF | Lian et al. (2013) |
| n = 47 |
| Quantafluor fluorescent autoantibody test kit. | Laskin et al. (1990) | |
| NHL |
|
| IIF | Altintas et al. (2008) |
|
| IIF—Hep2 cells | Guyomard et al. (2003) | |
| Leukemia | n = 196 |
| IIF | Wang et al. (2021) |
| n = 216 |
| IIFT | Sun et al. (2019) | |
| ANAs in autoimmune cutaneous diseases | ||||
| EPF |
|
| IIF | Nisihara et al. (2003) |
| PV |
|
| IIF | Saleh et al. (2017) |
| LP | n = 47 |
| IIF on Rat esophagus, Monkey esophagus, Hep-2 cells, and rat liver | Carrizosa et al. (1997) |
| n = 100 |
| IIF | Rambhia et al. (2018) | |
| Rosacea | n = 101-ANAs with titre 1:160 |
| IIF on Hep-2 | Woźniacka et al. (2013) |
The prevalence of ANAs non-rheumatic autoimmune diseases
| Disease | Analyzed groups | ANAs presence/detection of specific antibodies | ANAs detection method | Reference |
|---|---|---|---|---|
| PBC | n = 32 |
| IIF | Walker et al. (1965) |
| Psoriasis |
|
| IIF | Patrikiou et al. (2020) |
|
| ELISA | Singh et al. (2010) | |
| DMt1 |
|
| IIF | Heras et al. (2010) |
|
| IIF | Notsu et al. (1983) | |
| CD | n = 101 |
| IIF | Carroccio et al. (2015) |
| n = 161 |
| IIF | Almeida et al. (2019) | |
| Autoimmune thyroid disease |
|
| IIF | Lanzolla et al. (2023) |
|
| IIF using Hep-2 cells as substrate | Tektonidou (2004) | |
| n = 104 |
| IIF using Hep-2 cells as substrate | Torok and Arkachaisri (2010) | |
| PA |
|
| Indirect IIF | Morawiec-Szymonik et al. (2019) |
| AD | n = 1 |
| Serological screening | Yazdi et al. (2021) |
| 29-year-old with clinical features of acute Addisonian crisis and SLE |
| Serology screening | Godswill and Odigie (2014) |