Fig 1.
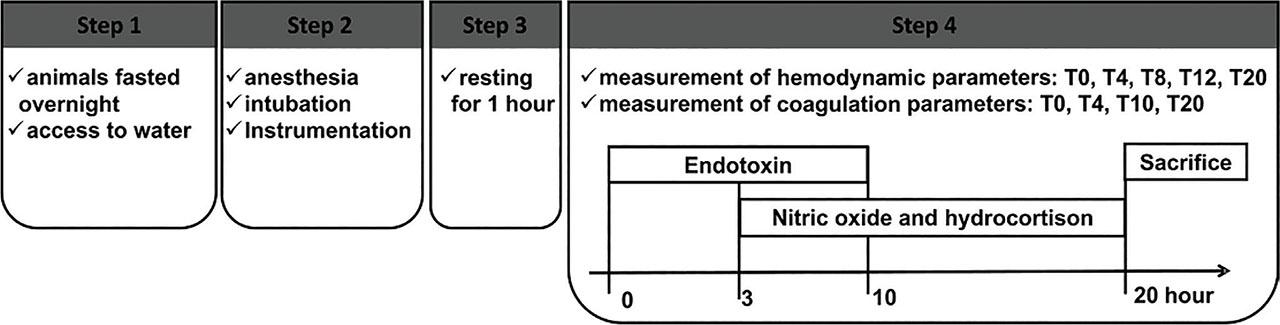
Fig 2.
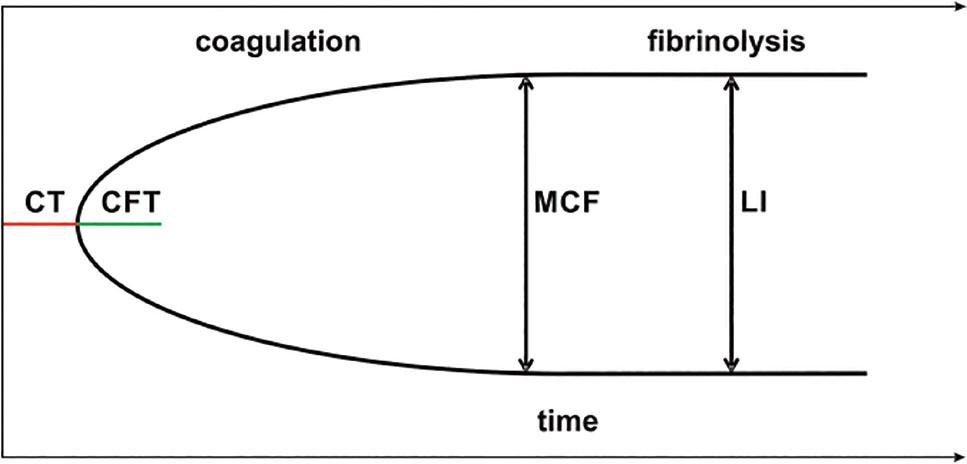
Fig 3.
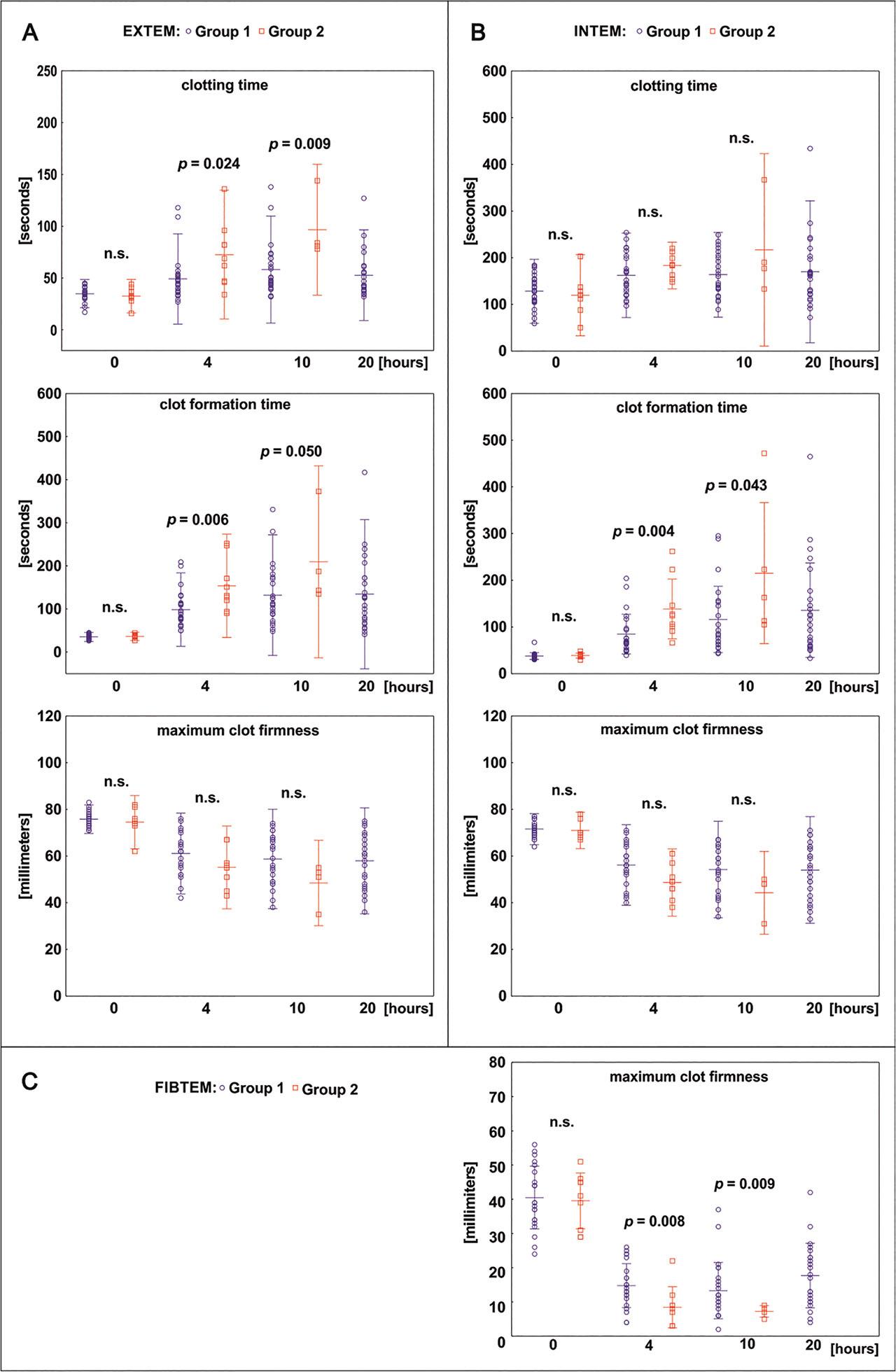
Fig 4.
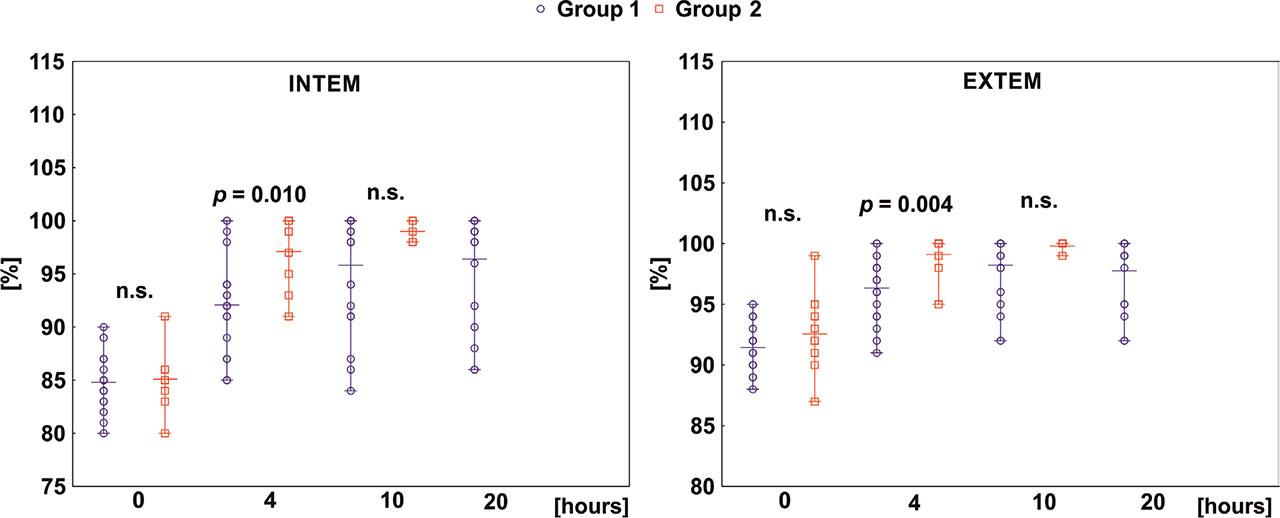
Fig 5.
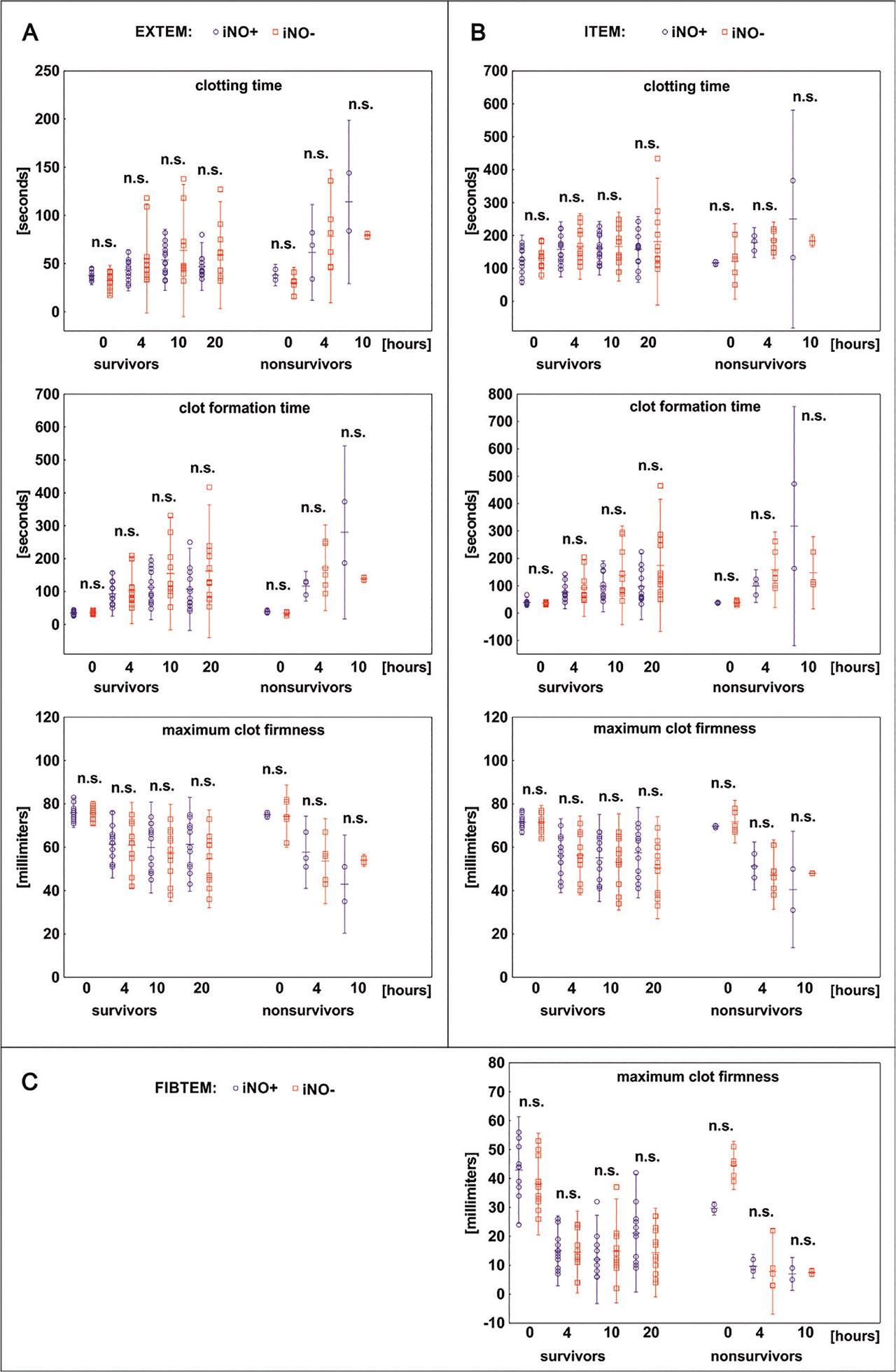
Changes in ROTEM parameters and other coagulation tests before (T0), during endotoxin infusion (T4 and T10), and after stopping of endotoxin administration (T20)
| Parameter | Time | All (N = 34) | Survivors (N = 25) | Non-survivors (N = 9) | p |
|---|---|---|---|---|---|
| EXTEM | |||||
| CT (s) | 0 | 34.2 ± 1.2 | 34.9 ± 1.3 | 32.5 ± 2.6 | 0.318 |
| 4 | 56.9 ± 4.4 | 49.1 ± 4.2 | 74.8 ± 9.5 | 0.024 | |
| 10 | 65.1 ± 5.4 | 58.2 ± 5.2 | 96.6 ± 12.2 | 0.009 | |
| 20 | 52.0 ± 4.3 | 52.0 ± 4.30 | |||
| CFT (s) | 0 | 35.4 ± 0.8 | 35.1 ± 1.1 | 36.3 ± 1.5 | 0.480 |
| 4 | 113.1 ± 8.9 | 98.5 ± 8.3 | 153.6 ± 19.9 | 0.006 | |
| 10 | 144.1 ± 14.5 | 132.0 ± 14.2 | 202.2 ± 43.3 | 0.050 | |
| 20 | 134.3 ± 17.6 | 134.3 ± 17.6 | |||
| MCF (mm) | 0 | 75.4 ± 0.6 | 75.8 ± 0.6 | 74.5 ± 1.9 | 0.623 |
| 4 | 59.3 ± 1.5 | 61.0 ± 1.6 | 54.4 ± 2.8 | 0.077 | |
| 10 | 57.1 ± 1.9 | 58.7 ± 2.1 | 49.6 ± 3.7 | 0.130 | |
| 20 | 57.9 ± 2.3 | 57.9 ± 2.3 | |||
| LI (%) | 0 | 91.7 ± 0.4 | 91.4 ± 0.3 | 92.5 ± 1.1 | 0.315 |
| 4 | 97.0 ± 0.4 | 96.3 ± 0.5 | 99.1 ± 0.5 | 0.004 | |
| 10 | 98.5 ± 0.4 | 98.2 ± 0.5 | 99.8 ± 0.2 | 0.237 | |
| 20 | 97.7 ± 0.6 | 97.7 ± 0.6 | |||
| INTEM | |||||
| CT (s) | 0 | 125.9 ± 6.1 | 128.1 ± 6.8 | 119.6 ± 13.5 | 0.570 |
| 4 | 168.8 ± 7.3 | 162.3 ± 9.2 | 186.1 ± 8.8 | 0.114 | |
| 10 | 173.4 ± 10.2 | 163.5 ± 8.8 | 223.0 ± 37,7 | 0.224 | |
| 20 | 170.2 ± 15.5 | 170.2 ± 15.5 | |||
| CFT (s) | 0 | 37.8 ± 1.1 | 37.6 ± 1.4 | 38.5 ± 1.7 | 0.353 |
| 4 | 98.7 ± 9.1 | 84.5 ± 8.3 | 138.4 ± 21.3 | 0.004 | |
| 10 | 133.1 ± 17.4 | 116.0 ± 14.5 | 215.2 ± 67.5 | 0.043 | |
| 20 | 135.7 ± 20.6 | 135.7 ± 20.6 | |||
| MCF (mm) | 0 | 71.4 ± 0.5 | 71.5 ± 0.6 | 71.0 ± 1.2 | 0.433 |
| 4 | 54.1 ± 1.4 | 56.1 ± 1.6 | 48.6 ± 2.3 | 0.057 | |
| 10 | 52.4 ± 1.9 | 54.2 ± 2.1 | 44.2 ± 3.4 | 0.872 | |
| 20 | 54.0 ± 2.3 | 54.0 ± 2.3 | |||
| LI (%) | 0 | 84.8 ± 0.4 | 84.8 ± 0.5 | 85.1 ± 0.9 | 0.734 |
| 4 | 93.4 ± 0.8 | 92.0 ± 0.8 | 97.1 ± 1.1 | 0.010 | |
| 10 | 96.4 ± 0.9 | 95.8 ± 1.0 | 99.0 ± 0.4 | 0.360 | |
| 20 | 96.4 ± 1.1 | 96.4 ± 1.1 | |||
| FIBTEM | |||||
| MCF (mm) | 0 | 40.2 ± 1.4 | 40.5 ± 1.7 | 39.5 ± 2.7 | 0.930 |
| 4 | 13.0 ± 1.1 | 14.7 ± 1.2 | 8.4 ± 2.0 | 0.008 | |
| 10 | 11.2 ± 1.1 | 12.1 ± 1.3 | 7.2 ± 0.85 | 0.009 | |
| 20 | 17.7 ± 2.0 | 17.7 ± 2.0 | |||
| Platelets (103/μL) | 0 | 415.7 ± 16.8 | 431.4 ± 18.6 | 372.1 ± 34.4 | 0.097 |
| 4 | 206.1 ± 22.3 | 229.5 ± 28.8 | 143.7 ± 17.5 | 0.045 | |
| 10 | 146.2 ± 14.5 | 153.9 ± 18.2 | 119.0 ± 8.4 | 0.434 | |
| 20 | 119.1 ± 16.7 | 119.1 ± 16.7 | |||
| Fibrinogen (mg · dL−1) | 0 | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.3 ± 0.4 | 0.482 |
| 4 | 2.0 ± 0.1 | 2.1 ± 0.1 | 1.7 ± 0.2 | 0.024 | |
| 10 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.2 ± 0.2 | 0.059 | |
| 20 | 2.2 ± 0.1 | 2.2 ± 0.1 |
The basic variables of the pigs before endotoxin infusion
| Parameter | All (N = 34) | Survivors (N = 25) | Non-survivors (N = 9) | p |
|---|---|---|---|---|
| EXTEM | ||||
| CT (s) | 34.2 ± 1.2 | 34.8 ± 1.3 | 32.5 ± 2.6 | 0.318 |
| CFT (s) | 35.4 ± 0.8 | 35.1 ± 1.1 | 36.3 ± 1.5 | 0.480 |
| MCF (mm) | 75.4 ± 0.6 | 75.8 ± 0.6 | 74.5 ± 1.9 | 0.623 |
| LI (%) | 91.7 ± 0.4 | 91.4 ± 0.3 | 92.5 ± 1.1 | 0.315 |
| INTEM | ||||
| CT (s) | 125.9 ± 6.1 | 128.1 ± 6.8 | 119.6 ± 13.5 | 0.570 |
| CFT (s) | 37.8 ± 1.1 | 37.6 ± 1.4 | 38.5 ± 1.7 | 0.353 |
| MCF (mm) | 71.4 ± 0.5 | 71.5 ± 0.6 | 71.0 ± 1.2 | 0.433 |
| LI (%) | 84.8 ± 0.4 | 84.8 ± 0.5 | 85.1 ± 0.9 | 0.734 |
| FIBTEM | ||||
| MCF (mm) | 40.2 ± 1.4 | 40.4 ± 1.7 | 39.5 ± 2.7 | 0.930 |
| Platelets (103 • μL–1) | 415.7 ± 16.8 | 431.4 ± 18.6 | 372.1 ± 34.4 | 0.097 |
| Fibrinogen (mg • dL–1) | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.3 ± 0.4 | 0.482 |