Background & Objectives
Health risk assessment (HRA) is a comprehensive approach that entails identifying, evaluating, and prioritising potential health risks and vulnerabilities for individuals and populations and identifying possible measures to reduce or mitigate their effects.
Deploying appropriate HRA strategies is a cornerstone for population risk stratification and constructing the corresponding population risk pyramid. It has become essential for informing health policy decisions, allocating resources, benchmarking, implementing preventive strategies and selecting appropriate healthcare services [123]. Likewise, in the clinical arena, HRA is a building block for generating predictive models to support clinical decision-making [45]. Indeed, population-based, and clinically-oriented HRA approaches are complementary elements needed to efficiently adopt integrated patient-centred care strategies. Deploying and adopting well-planned HRA strategies constitute an obligatory step toward the maturity of precision medicine [6].
Moreover, the momentum propitiated by the continuous progress in digital technologies for data capture and management, artificial intelligence and the advances in medical sciences are shaping novel and stimulating scenarios for health promotion and care and positioning predictive medicine at the forefront [789]. Despite the promising potential of HRA, there is a noticeable gap between its benefits and its current application, attributed to several limitations, including the utilisation of suboptimal risk assessment tools, the insufficient engagement of health professionals, the application of ineffective or inexistent deployment strategies, and unresolved ethical and regulatory issues [1011].
The Joint Action on implementation of digitally enabled integrated person-centred care (JADECARE) [12], an ongoing initiative launched to face the challenges of the transformation of health in the European Union, has included HRA as a strategic block to transfer from four original Good Practices (oGPs) to other twenty-one European regions participating as Next Adopters (NAs).
The central aims of JADECARE are to reinforce the capacity of health authorities for successfully addressing all the crucial aspects of health system transformation, in particular, the transition to digitally enabled, integrated, person-centred care, and to support the best practice transfer from the oGPs to the corresponding NAs. The current integrated case reports:
The evolution of the HRA strategy in one of the JADECARE oGPs (i.e., Catalonia, ES) from 2011–2020.
The description of the implementation process followed to transfer a population-based risk assessment tool from Catalonia to two NAs: Marche region (IT) and Estonia (EE).
The lessons learnt in the form of recommendations to foster the adoption of enhanced health risk assessment across the EU.
The paper aims to identify key barriers and facilitators for effective adoption across Europe of population-based health risk assessment at the regional/country levels and formulate proposals facilitating the articulation between population-based and clinically-oriented HRA.
Description of the care practice
HRA in Catalonia: 2011–2020
The 2011–2015 Catalan Health Plan [13] fostered key achievements in digital health transformation, the progressive implementation of person-centred integrated care services and the adoption of an initial population-based HRA strategy. This transitory HRA strategy was based on the commercial solution “Clinical Risk Groups” (CRG) [14] from vendor 3M™ and oriented toward modelling healthcare costs for resource allocation and benchmarking.
This initial HRA strategy laid the groundwork for a population-based risk stratification program with a case-finding approach focused on preventing adverse health events, managing high-risk chronic patients, and early detecting end-of-life patients [15]. This HRA approach acknowledged the close relationship between frailty and multimorbidity while recognizing their distinct nature as independent risk factors. Additionally, it introduced specific scales for the evaluation of each factor individually. The clinical complexity level was assigned based on multimorbidity scoring, transitively using CRGs, and the clinical judgment of primary care physicians. Complex patients were classified into two groups: complex chronic patients (CCP), approximately 4% of the population, and advanced chronic patients (ACP), representing approximately 1% of the population with limited life expectancy. Specific community-based management plans, aiming at integrating health and social care, were defined for these two categories of patients [15]. An overall description of the care model for people with frailty and multimorbidity in Catalonia, tested during the 2011–2015 Health Plan, has been recently reported in [16].
The need for refining the assessment of the multimorbidity burden prompted the creation of the Adjusted Morbidity Groups (AMG) [1718], a new morbidity grouper that reflects patients’ disease burden in terms of the number and complexity of concomitant disorders through a disease-specific weighting deduced from statistical analysis based on mortality and the utilisation of healthcare resources. Two main factors triggered the development of the AMG. Firstly, awareness of HRA as one of the pivotal elements for scalability of integrated care [161012]. Also, the identification of weaknesses in the existing morbidity groupers [111].
The AMG tool was jointly launched in 2015 by the Spanish Health Ministry and the Catalan public health commissioner (CatSalut). The rationale behind the development and adoption of the AMG in Spain, as part of the Chronicity Strategies and risk stratification tools, was reported in [19].
A significant achievement was the development of a dashboard to monitor the population’s multimorbidity burden and the use of healthcare known as Modules for Monitoring Quality Indicators (“Moduls pel Seguiment d’Indicadors de Qualitat”, MSIQ) [20], which generates and displays customised key performance indicators (KPIs) with aggregated data to inform health policy decisions, benchmarking, and governance. On the clinical side, the AMG scoring of the patient is currently displayed in the workstation of the primary care physicians and the shared clinical history [21] as a support tool in the clinical setting.
During the 2016–2020 Catalan Health Plan [22], the utilisation of AMG for HRA purposes was validated in different regions of Spain, covering a population of approximately 38 million citizens and showing good transferability in all cases [19]. This period witnessed three significant advancements in Catalonia’s HRA strategy:
The execution of several studies testing the contribution of the AMG in different HRA settings, such as the identified risk factors during the SARS-CoV-2 pandemic [2324], the refinement of tools for resources allocation [25262728], the analysis of the effect of the multimorbidity burden in patients with chronic obstructive pulmonary disease (COPD) [29], and assessing the use of AMG in complex clinical predictive models for short-term clinical outcomes predictions after hospital discharge [45].
The creation of the Catalan Health Information System Master Plan published in 2019 [30] established the basis for the transformation to a new digital-health paradigm based on a knowledge-driven platform and adopting the Open-EHR standard as a reference.
The development and internal validation of the Queralt indices [3132] to characterise the complexity of hospitalisation episodes, combining information on the principal discharge diagnosis, pre-existing comorbidities, in-hospital complications and all the procedures performed during hospitalisation.
Figure 1 offers a comprehensive view of the AMG system, illustrating its workflow from the initial data input to the final application in health risk assessment. Panel A details the essential input variables required for computing the AMG, which include disease diagnostic codes as only variable needed for the calculations. Additionally, it incorporates sex and date of birth, which are used for ensuring data quality and validating the diagnoses. Lastly, the health area, which is primarily employed to aggregate the results. Panel B reveals the outputs of the AMG algorithm, highlighting key metrics such as the total number of chronic diseases, the count of affected organic systems, and a battery of presence/absence markers for highly relevant chronic conditions. Also, the AMG category, which systematically groups patients based on the quantity and type of their chronic conditions, supplemented by the morbidity burden score. This score reflects the patient’s complexity, quantifying the number and severity of concurrent disorders with a disease-specific weight derived from statistical analysis, considering the impact on healthcare resources. Panel C showcases the potential application of AMG system in health risk assessments, emphasizing its role in the classification, stratification, and identification of patient needs and risks.
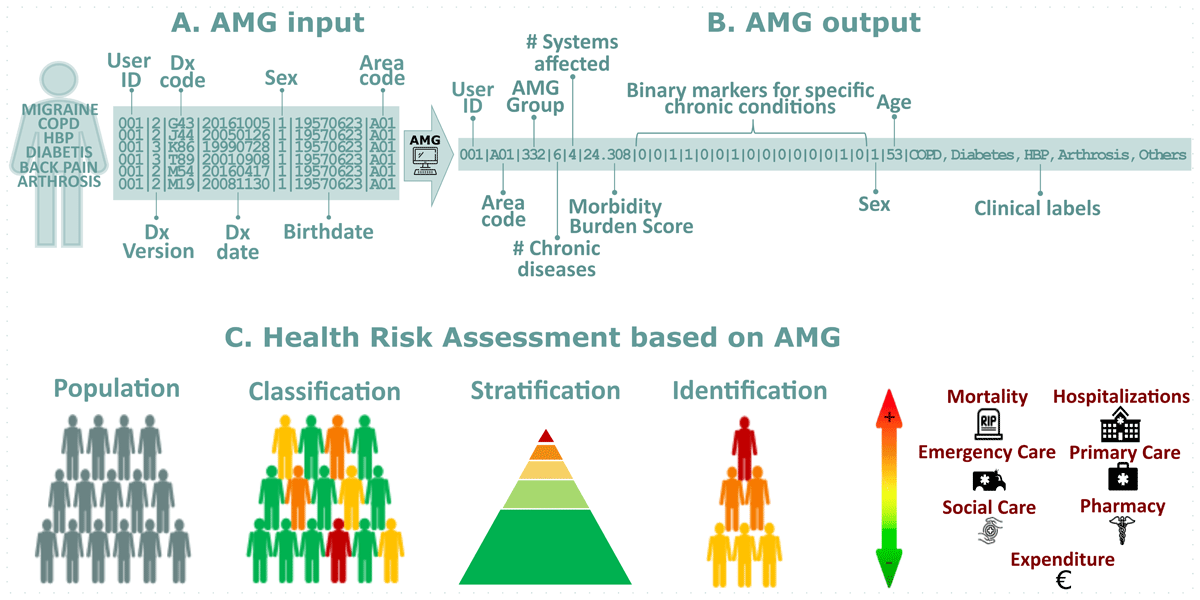
Figure 1
Panel A – AMG input: Required input variables to compute AMG; Panel B – AMG output: Output variables of the AMG algorithm. *Binary markers (presence/absence) of 15 chronic conditions (from left to right): diabetes mellitus, heart failure, chronic obstructive pulmonary disease, high blood pressure, depression, HIV/AIDS, chronic ischemic heart disease, stroke, chronic kidney disease, cirrhosis, osteoporosis, arthrosis, arthritis, dementia, chronic pain; Panel C– Health Risk Assessment based on AMG: The AMG scoring allows for three key actions: Classification: The population is categorised into specific groups based on their morbidity statuses, such as healthy, pregnancy and labour, acute disease, chronic disease in 1–4 systems, or active neoplasia, which are also divided into five degrees of severity. Stratification: Each individual can be assigned a complexity score that reflects the care needs that people may have based on their health problems. Identification: Individuals with specific major chronic health problems can be identified, which helps track people with more complex care needs.
Pre-implementation (October 2020 – September 2021)
Within JADECARE, the AMG was transferred to the Marche region and Estonia. The Marche region has a regionally based healthcare system, providing universal coverage to 1,480,839 citizens of which 25.4% are 65 years and older. Life expectancy is of 81 years for men and 85.2 for women. In comparison, Estonia has compulsory solidarity-based health insurance, financed by the health insurance budget through the Estonian Health Insurance Fund, covering 1,322,765 citizens, 29.0% aged 65 years and older. Life expectancy at birth is 72.8 years for men and 81.4 for women.
In Estonia, the initial implementation site for the AMG transfer was chosen was Viljandi County with approximately 30 general practitioners and one general hospital providing specialist care for around 50,000 inhabitants.
Table 1 describes the context and the trigger that motivated the adoption of the AMG in the Marche region and Estonia, alongside the local aims of each NA and the main challenges identified by the oGP leaders. Specific Local Action Plans were designed to fulfil the needs of each NA, as reported in detail in the Supplementary Material. The pre-implementation phase concluded once the implementation feasibility study was successful in each site.
Table 1
Summary of the pre-implementation process, including the context and trigger, the aims, the baseline situation, and the challenges faced in the Marche region and Estonia.
| MARCHE REGION | ESTONIA | |
|---|---|---|
| Context and trigger |
|
|
| Aims |
|
|
| Baseline situation | The Regional Healthcare Administrative Databases (HADs), used at regional and national levels to monitor healthcare system expenditures and performance, gather information on healthcare services provided to citizens (e.g., hospitalisations, emergency-urgency, homecare, exemption codes, etc.). Common standard models and coding systems, such as DRGs [33] and ICD-9-CM [34], are used across all regions. However, each HAD has its unique structure, unit level, content, and rules for data input. Data linkage across HADs is facilitated using a unique anonymised patient ID code. | The Estonian Health Insurance Fund claims database was used for model data input. In Estonian universal healthcare and single payer model this database entails almost all medical care claims in the country. The database follows a single standard with ICD-10-CM [35] coding, DRGs. Data linkage and access was granted though ethics committee approval and is not easily available as standard. Data was analysed in anonymised format using unique patient ID codes. All AMG analyses were performed by the regional medical authorities under the ethics committee approved application with support from the oGP. |
| Challenges |
|
|
Results of the feasibility analysis
To assess the transferability of the AMG tool, independent feasibility tests were conducted in each of the adopting regions. To perform the AMG feasibility analysis in the Marche region, fully anonymized information on disease diagnostics from 2015 to 2019 was extracted from three independent databases: the hospital admissions, the emergency department, and the exemption codes for chronic and rare diseases databases. Integrating all the medical information resulted in 5,939,199 diagnostic codes associated with 1,367,181 citizens. In Estonia, the AMG feasibility study was conducted with 25,930 diagnostic codes related to 4,765 citizens treated in Viljandi Hospital in 2018.
The feasibility analysis evaluated the comprehensiveness of the clinical information accessed and the discrimination capacity of the AMG algorithm to identify high-risk individuals allocated in seven morbidity groups: healthy, pregnancy, acute disease, 1 or 2–3 or ≥ 4 chronic diseases, and active neoplasia.
When evaluating the feasibility study, it is imperative to consider the databases’ dual approach and purpose. The Marche database is a population health database [36], built for informing health policies, benchmarking, and supporting decision-making processes. On the other hand, the Viljandi database follows a population medicine approach [36], assembling patient registries to screen candidates for a clinical program geared towards preventing hospitalisations for elderly patients with concomitant chronic conditions.
The feasibility tests were deemed successful if the following conditions were met:
The algorithm effectively discriminated the patients with different risk profiles within specific age and gender groups, especially in older individuals more susceptible to multimorbidity.
The algorithm distinguished between the seven AMG disease groups. It’s worth noting that the relative frequencies of these groups may differ depending on the database’s nature.
The results showed a positive correlation between the clinical complexity of the AMG disease groups and the disease burden evaluated using the AMG Morbidity Burden Index.
The feasibility analysis results are shown in Figure 2. In Panel A, the distribution of the five subgroups of complexity is depicted according to age and gender. As expected, the demographic characteristics of the population treated in Viljandi utilised in this feasibility study differ from the Viljandi county’s population. Overall, the AMG algorithm proficiently distinguished patients with varying risk profiles in specific age and gender categories. Panel B shows the distribution of the seven AMG morbidity groups’ complexity level, represented by the average Morbidity Burden Score. In the Marche region and Estonia, roughly 50% of the population suffers at least one chronic disease and 20% developed multimorbidity, while 5% has an active neoplasm. Notably, the analysis of the population treated in Viljandi Hospital revealed a higher fraction of citizens with acute morbid conditions due to the hospital nature of the database. When comparing the relative distribution of AMG disease groups between the adopting regions and Catalonia, notable disparities emerge in terms of the prevalence of patients with multimorbidity. These variations can be attributed to the availability of primary care registries, which is a condition exclusively fulfilled in the Catalan context. This underscores the critical importance of integrating health data from diverse levels. The complexity of the disease groups and the AMG Morbidity Burden Score showed a strong positive correlation, being slightly higher in the Marche region due to the exhaustivity of the diagnostic records at different healthcare levels and the increased length of the study period.
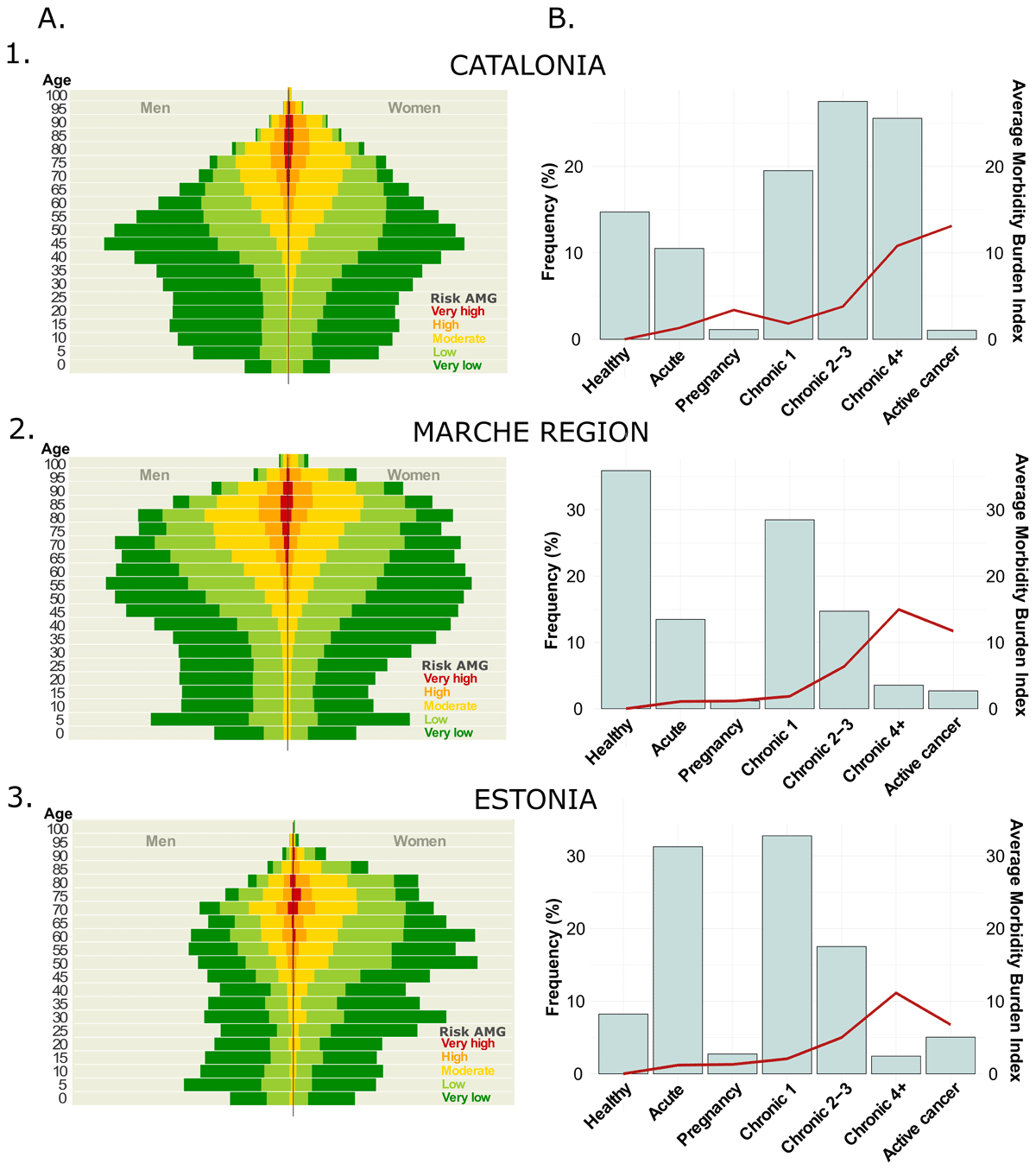
Figure 2
Results of the feasibility analysis: in Catalonia (1) and the adoption regions, Marche Region (2) and Estonia (3). Panel A – AMG risk distribution: itemised by age and gender; Panel B – AMG disease groups: distribution of the seven AMG morbidity groups (bars): healthy, pregnancy, acute disease, 1 or 2–3 or ≥4 chronic diseases, and active neoplasia, and their average Morbidity Burden Score (line).
Given that the outcomes of the feasibility analysis satisfied the abovementioned criteria, which include the algorithm’s capability to differentiate patients based on risk profiles, its success in identifying the seven distinct AMG disease groups, and the observed positive correlation between clinical complexity and the AMG Morbidity Burden Index, the oGP specialists determined that the databases from the adopters, namely the Marche region and Estonia, were sufficiently mature. This maturity signifies their preparedness to proceed with the AMG system’s implementation and the readiness to expedite the implementation of the AMG.
Implementation (October 2021–January 2023)
On October 2021, the Local Action Plans (LAPs) in Marche and Estonia were already available, and the corresponding NA Working Groups were prepared to undertake two Plan-Do-Study-Act (PDSA) cycles [37]. The details of the PDSA cycles are reported in the Supplementary Material. A major midterm milestone of the implementation phase was the HRA Thematic Workshop, held in Viljandi Hospital on 14–15 June 2022. The workshop delimitated the bases for deploying population-based HRA strategies focused on AMG and the Queralt indices. A recording of the session can be found in the Supplementary Material.
Marche
Implementation achievements – 1) dynamic regional dataset preparation merging information from different existing data sources, 2) data cleaning and automatization of the generation of the regional dataset, 3) elaboration and analysis of the risk assessment pyramid at regional level demonstrating association between GMA scoring and local use of resources, 4) preparation of the logistics for local sustainability of the setting; and 5) design of the regional dashboard to facilitate regional health governance. The technicalities of the implementation and the assessment of the implementation process will be reported in a forthcoming paper.
Sustainability Action Plan – 1) to complete the integration of the tool into the regional IT infrastructure, adding further healthcare databases and defining supportive actions to improve the quality and completeness of healthcare data. 2) to implement the dashboard in computational, technical, and graphical terms, adding maps aimed to visualize healthcare services adjusted to social-health care planning regulations and integrating it in the regional IT infrastructure. 3) to promote the use of the HRA tools by regional and clinical managers and share our experience for the discussions on the secondary use of health data.
Estonia
Implementation achievements – 1) Generating a protocol, already approved by the local Ethics committee for a pragmatic randomized control trial (n = 1000), to test effectiveness and value generation of an integrated care intervention to prevent hospitalizations targeting community-based patients with high-risk of admissions and enhancing transitional care post-discharge to reduce early readmissions (PAIK 2022–2025). 2) AMG will be used as inclusion criteria and to modulate the characteristics of the intervention and the Queralt indices will be employed to characterize hospitalization episodes contributing to personalize transitional care after discharge.
Sustainability Action Plan – 1) to successfully execute the PAIK 2022–2025 project using AMG and Queralt indices as risk assessment tools. 2) to generate sound proposals for innovative reimbursement modalities. 3) to achieve country-wide scalability of risk prediction approaches based on AMG and Queralt. 4) to initiate a debate on the scalability of integrated care services involving innovative reimbursement modalities in Estonia.
Perspectives beyond implementation
The section describes the status of the HRA in Catalonia and briefly reports the overarching analysis of the process of transference to the two NAs, leading to recommendations for the generalisation of the case practice at the European level.
Evolution of the Catalan oGP during JADECARE
The 2020 Catalan Health Information System Master Plan has sparked ongoing technological innovations that offer significant opportunities in predictive modelling aiming at supporting clinical decision-making for healthcare professionals and provide patients with decision support tools to empower self-management [383940]. Also, specific initiatives have been launched to enhance transitional care and reduce early readmissions after hospital discharge [45]. Moreover, efforts are devoted to the practicalities of adoption of such predictive modelling tools in real-world settings, involving: 1) training and continuous evaluation of clinical decision support systems embedded into integrated care services, 2) pragmatic use of implementation science tools to foster engagement of health professionals, and 3) ethical and regulatory aspects including refinement of the regional PADRIS program [41] for secondary use of health data.
In Catalonia, research, and innovation in HRA are currently focused on two target areas. Firstly, to address unmet needs associated with enhanced predictive modelling considering four potential sources of variables: 1) clinical information, 2) registry data with a population health approach, 3) patients’ self-reported information (PROMs and PREMs) and self-monitoring data, and 4) biomedical research data, as defined in [1]. A key learning from the different studies done using the AMG algorithm since 2015 is that multimorbidity has a central explanatory role in health risk assessment, more than classical variables like age, which prompts the inclusion of the AMG scoring as a fix covariate in multisource predictive modelling.
A second field of research interest is the potential evolutions of AMG incorporating information on patients’ disease trajectories [4243]. A recent study [44] revealed that this approach provides promising prospects to support tailored epidemiological studies, enabling targeted interventions for particular patient groups. Additionally, integrating the probabilities of disease-disease co-occurrence into the evaluations could assist in assessing the risk of developing certain disease conditions. Although these novel computational developments are still far from being applicable in clinical settings, they present exciting prospects or precision medicine in patients with NCDs.
Overarching analysis
The case practice identifies the process of transference and adoption of the AMG algorithm and a site-tailored dashboard using aggregated data as the two key components necessary for establishing fundamental HRA functionalities.
While Marche adopted a population-health approach considering the use of healthcare resources from the entire geographical area, Viljandi Hospital in Estonia implemented a more limited regional population-medicine orientation with a single hospital as a local integrator and lead in a regional health improvement initiative. As depicted in Figure 2, the differences in terms of sources and composition of input data had significant impacts on the distribution of AMG scoring among Catalonia (Figure 2 – Panel 1, population-health data from all healthcare tiers), Marche (Figure 2 – Panel 2, population-health data with poor representation of primary care) and Viljandi Hospital (Figure 2 – Panel 3, population-medicine approach based on hospital information). Accordingly, population-health databases, as those used in Marche region, must comprise information on disease diagnostics from all individuals within the region, gathered from various tiers of the healthcare system (e.g. primary care, community, hospital, etc.). It is expected to find a significant fraction of the healthy population and cases with transitory health problems, both concentrated in the youngest fraction of the population. The remaining population is expected to suffer at least chronicity and many of them to develop multimorbidity; among them is expected to find a small fraction of complex chronic patients.
On the other hand, if the analysis is conducted following a population medicine approach, as done in Viljandi, the sample should be representative of the population treated at the hospital during the period assessed, both in terms of demographic parameters and the clinical profiles of the patients. In light of this, a highly biased population is expected to be older than the hospital’s catching area average. Also, a substantial increase in the frequency of patients experiencing mainly acute and chronic conditions is anticipated.
Consequently, the composition of the input datasets plays a crucial role in modulating both purposes and suitability of adopting HRA strategies. In the current case practice, Marche’s approach was adequate to cover health policy aspects, resources allocation, benchmarking, and governance at the regional level, even though limitations due to GDPR constraints at the Italian level should be acknowledged. In contrast, the HRA orientation adopted by Viljandi Hospital was beneficial in the design process of the PAIK2 protocol (2023–2025), aiming at generating evidence of the effectiveness of an integrated care intervention to prevent hospitalisations in high-risk patients and transitional care. It is of note that the process of developing the case practice in the two sites has generated knowledge and skills that will be needed to elaborate future site-customised comprehensive HRA strategies, as described in the case of Catalonia.
In summary, the essential maturity requirements for sustainable HRA strategies are: 1) to achieve solid political commitment at the regional/country level fostering necessary interactions, top-down and bottom-up, among key stakeholders; 2) to have an essential digital maturity at the site level to satisfactorily pass the feasibility analysis; 3) to overcome potential limitations due to local application of GDPR; 4) to use highly applicable implementation science tools fostering engagement of all stakeholders, health policy managers and health professionals, during the process elaboration and deployment of the local HRA strategy; and 5) to develop/adopt a regulatory framework for secondary use of health data, needed for business intelligence and the elaboration of computational modelling for clinical applications. The five maturity requirements briefly described above have been identified through the interactions with all NAs during JADECARE’s lifetime, specifically from the Thematic Workshops and the Key Lessons learnt during the post-implementation period. As a summary, Table 2 depicts the steps identified during the transference process to define a roadmap for adopting site-customised HRA strategies.
Table 2
Checklist of key steps for site adoption of Health Risk Assessment (HRA).
| KEY STEPS | DESCRIPTION |
|---|---|
| 1. Scope definition | Identify the purpose, focus/use and ambition of the HRA initiative |
| 2. Source population | Population-health or Population-medicine. For each option, identify specificities of the source population |
| 3. Planned updates | Periodicity of source data update (i.e. yearly basis) |
| 4. Model (Morbidity grouper) | Morbidity grouper selected: AMG, CRG, ACG, others |
| 5. Input variables (Figure 1) | Minimum variables of the morbidity grouper plus additional variables selected for inclusion in the HRA modelling. |
| 6. Data source(s) (data sources and ownership have technical/managerial implications) |
|
| 7. Predictive modelling | Identify statistical methods, Machine Learning, Deep Learning, etc… |
| 8. Output variables(Figure 1) | Variables generated by the predictive modelling approach |
| 9. Feasibility assessment | Includes characterization of site maturity and preliminary testing of the HRA tools to ensure minimum quality before implementation |
| 10. Technological logistics | The complexity of the digital setting is closely related to the characteristics of the data sources and ambition of the HRA strategy. It requires assessment of sustainability over time in terms of technological and human resources. |
| 11. Initial assessment of the core predictive modelling | Quality assessment to be carried out immediately after deployment to define further steps leading to a sustainable HRA program |
| 12. Quality assurance program | Continuous quality assurance checking of the HRA program after sustainable adoption |
| 13. Dashboard preparation | Initial identification of key performance indicators (KPIs) to be monitored after adoption, as well as subsequent enrichment of the dashboard with novel KPIs as required. |
| 14. Stakeholders’ engagement | Highly applicable implementation research tools in place to foster engagement of users. The professional profiles will depend on the focus of HRA: policy makers, managers, clinical professionals, etc… |
| 15. Additional functionalities & Roadmap for further developments | Successful HRA adoption leads to initiatives to expand use and ambition requiring development of additional functionalities (predictive modelling) and definition of a roadmap for further developments either in the health policy area, management, clinical applications and/or research-innovation. |
Moreover, using the HRA tools described in the case practice as open-source software and articulating public-private collaborations supporting productive interactions and networking among sites are essential to speed up transferability and large-scale adoption of HRA strategies at the European level.
Discussion
The current paper is filling an existing gap in information on building up an HRA strategy at the regional/country level, as described for Catalonia, and its potential for transferability to other sites. Moreover, the Marche and Viljandi Hospital process generated a helpful checklist for generalising such transference to other sites across Europe.
The case practice has also recognised the convenience of further collaboration among regions beyond JADECARE to keep progressing toward mature HRA strategies contributing to paving the way for precision medicine. Moreover, the use of the current and future available algorithms (i.e. AMG, Queralt, etc.), and dashboards, as open-source software, as well as the provision of consultancy services supporting future next adopters, were identified as core elements to foster the adoption of efficient HRA strategies across Europe. To this end, the elaboration of a survey aiming at characterizing the key steps for site adoption of HRA (Table 2) in all JADECARE’s sites is strongly encouraged. The main aim is to identify the status and needs of all NAs regarding HRA. The information from the survey gathered can contribute to refining a large-scale implementation protocol to be customised at the site level beyond the project lifetime.
Lessons learned
The analysis of the HRA strategies in Catalonia (2010–2023), as well as the process of transference and adoption of the AMG in Marche (IT) and Viljandi Hospital (EE) within JADECARE, 2020–2023, generated the following key learnings aiming at fostering large-scale adoption of HRA across Europe:
Adopting comprehensive HRA strategies, including multimorbidity weight (AMG scoring) as a central component, constitutes a key element fostering health systems transformation toward value-based healthcare with a patient-centred approach.
Population-based and clinically-oriented HRA must be considered complementary and highly synergistic. However, ethical and regulatory aspects for secondary use of health data must be appropriately assessed and locally implemented.
Transferability of AMG across sites with diverse source data models is feasible, provided that the key input variables are available, the source population is well-characterized, and adequate data management with quality assurance over time is in place.
The current analysis identified the relevant steps to be considered for a generic protocol aiming at the implementation of HRA at a regional level.
Short-term elaboration of a map describing both maturity levels and needs for HRA adoption in the twenty-one JADECARE sites could provide the necessary information to define a roadmap leading to large-scale implementation of HRA across Europe.
Conclusions
The current study describes the evolution and the potential of the HRA strategy adopted in Catalonia since 2010. It also illustrates the transferability of the AMG algorithm in two different scenarios. Moreover, the case practice identified relevant barriers/facilitators modulating the adoption of HRA at regional level and elaborated a set of key steps to be considered for site deployment of HRA. Last, but not least, this work proposes initial steps to define a roadmap aiming at fostering large-scale implementation of HRA across Europe.
Additional File
The additional file for this article can be found as follows:
Supplementary Material
Toward adoption of health risk assessment in population-based and clinical scenarios. DOI: https://doi.org/10.5334/ijic.7701.s1
Appreviations
ACP: Advanced Chronic Patients
AMG: Adjusted Morbidity Groups
CCP: Complex Chronic Patients
COPD: Chronic Obstructive Pulmonary Disease
CRG: Clinical Risk Groups
GDPR: General Data Protection Regulation
HAD: Healthcare Administrative Database
HRA: Health Risk Assessment
KPI: Key Performance Indicator
LAP: Local Action Plan
MSIQ: Modules for Monitoring Quality Indicators (“Moduls pel Seguiment d’Indicadors de Qualitat”)
NA: Next Adopter
NCD: Non-Communicable Disease
oGP: Original Good Practice
PDSA: Plan-Do-Study-Act
PREM: Patient Reported Experience Measure
PROM: Patient Reported Outcome Measure
Reviewers
Miquel À. Mas, Direcció Clínica Territorial de Cronicitat Metropolitana Nord, Institut Català de la Salut and Department of Geriatrics, Hospital Universitari Germans Trias i Pujol, Badalona, Catalonia, Spain.
Roberto Nuño Solinís, IJIC Board member.
Funding Information

This research was funded by JADECARE project- HP-JA-2019 – Grant Agreement nº 951442 a European Union’s Health Program 2014–2020.
Competing Interests
The authors have no competing interests to declare.
Author Contributions
RGC, IC and JR conceptualized and led the study, with institutional support and guidance from the Catalan Health Service, PP and JPJ. DM, EV and MC provided expertise on the transference of AMG across regions and oversaw feasibility studies. RP and MdM spearheaded the adoption of AMG in the Marche region, coordinating the local MP, GF, and FB team. Similarly, MK led the implementation of AMG at Viljandi Hospital, coordinating efforts with local team members SH and AA. RGC and JR drafted the initial manuscript, including text writing and figure generation. All authors rigorously reviewed and approved the final manuscript, taking full responsibility for the work’s accuracy and integrity.
