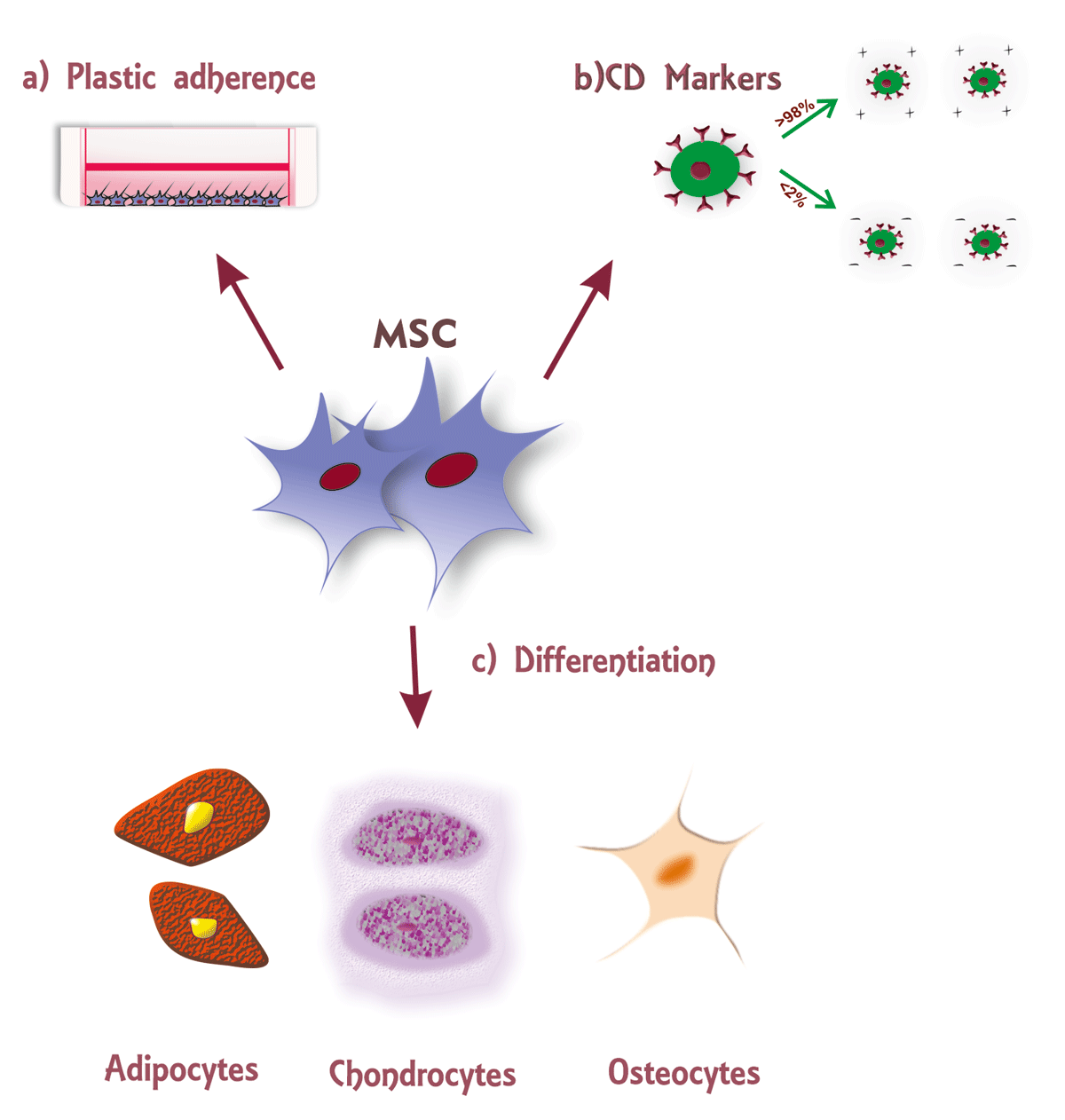
Figure 1
According to the International Society for Cellular therapy (ISCT) statement, Mesenchymal Stromal Cells defined as a) adherent to the plastic surface b) express Cluster Differentiation antigen, >98% positive for CD105, CD73, and CD105, and <2% negative for CD45, CD34, CD14 or CD11b, CD79alpha or CD19, HLA-DR surface molecules c) should differentiate into adipocytes, chondrocytes, and osteocytes.
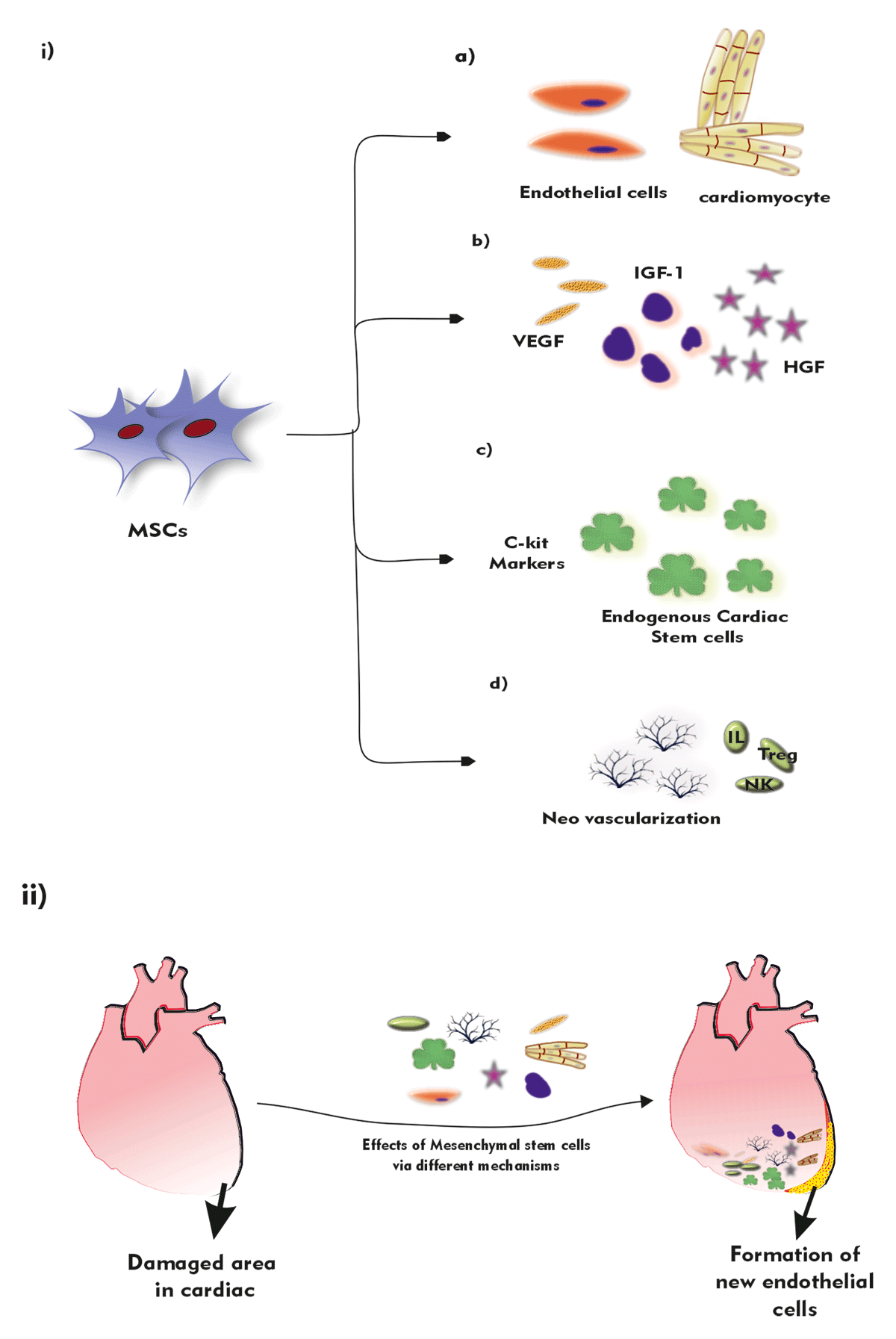
Figure 2
Mechanism of action of Mesenchymal stem cells: I) MSC cells can a) transdifferentiation into endothelial cells and cardiomyocytes b) promote paracrine signals such as Vascular endothelial growth factor (VEGF), Hepatic growth factor (HGF), and Insulin-like growth factor (IGF) c) proliferate endogenous cardiac stem cells with C-Kit markers d) stimulate the growth of neovascularization and immunomodulation. II) Overview of formation of new endothelial cells, reduction in infarct size of damaged cells via various mesenchyme effects.
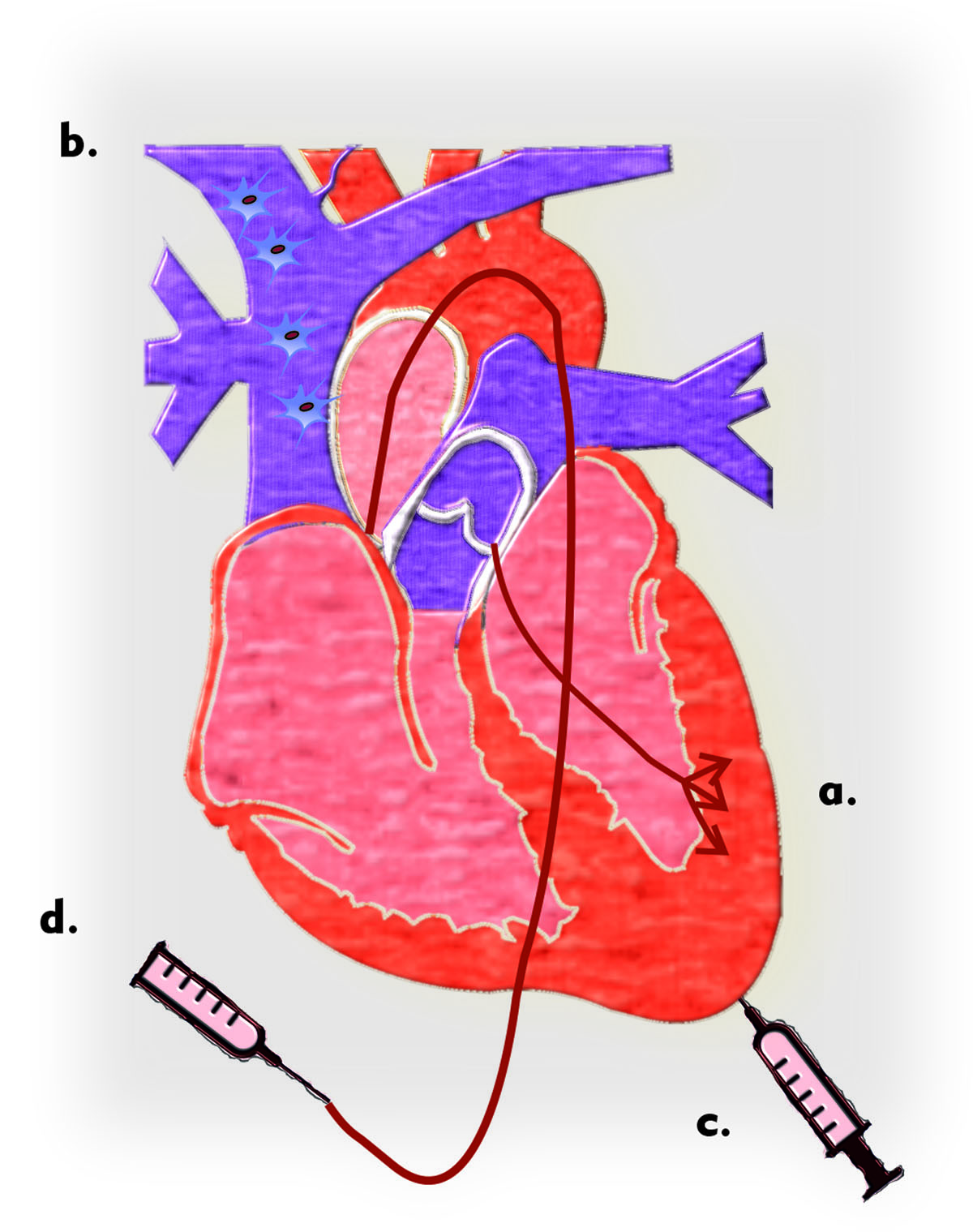
Figure 3
A diagram of various cell therapy approaches tested with MSCs is shown: a) transendocardial stem cell injection, b) peripheral intravenous infusion, c) intramyocardial delivery by injection catheter, and d) delivery via an intracoronary artery.
Table 1
List of few completed clinical trials of MSC therapy in ischemic heart disease.
| STUDY | n | CELL SOURCE | CONDITION | DESIGN | DELIVERY | ID NUMBER |
|---|---|---|---|---|---|---|
| Chronic ischemic heart disease: | ||||||
| TRIDENT | 30 | Allogeneic BM | Ischemic CM | Phase II | TESI | NCT02013674 |
| TAC-HFT | 65 | Autologous BM | Ischemic MI | Phase I/II | TESI | NCT00768066 |
| PROMETHUS | 9 | Autologous BM | Ischemic MI | Phase I/II | IM | NCT00587990 |
| MESAMI – I | 10 | Autologous BM | Ischemic CM | Phase I/II | TESI | NCT01076920 |
| MyStromal Cell | 60 | Autologous ADSC | Ischemic CM | Phase II | IM | NCT01449032 |
| Paulo et al | 10 | Autologous BM | Ischemic CM | Phase I/II | IC | NCT01913886 |
| Kyriakos et al | 11 | Allogeneic BM | Ischemic CM | Phase II | IM | NCT01759212 |
| POSEIDON | 31 | Autologous/Allogeneic BM | Ischemic CM | Phase I/II | TESI | NCT01087996 |
| Vrtovec et al | 110 | Autologous BM | Ischemic dilated CM | Phase II | IC | NCT00629018 |
| PRECISE | 27 | Autologous ADSC | Ischemic CM | Phase I | IM | NCT00426868 |
| Perin et al | 60 | Allogeneic BM | Ischemic CM | Phase II | TSEI | NCT00721045 |
| Acute Myocardial infarction: | ||||||
| SEED-MC | 80 | Autologous BM | Acute MI | Phase II/III | IC | NCT01392105 |
| Lian et al | 160 | Allogeneic UC | ST-elevation MI | Phase II | IC | NC00114452 |
[i] Abbreviations: BM-Bone Marrow; ADSC- Adipose-Derived Stem Cell; UC-Umbilical Cord; CM-Cardiomyopathy; MI-Myocardial Infarction; TESI- Transendocardial stem injection; IM- intramyocardial; IC-intracoronary; MSC-Mesenchymal stem cells.
Table 2
Summary of on-going clinical trials of MSC therapy in ischemic heart disease.
| STUDY | n | CELL SOURCE | CONDITION | DESIGN | DELIVERY | ID NUMBER |
|---|---|---|---|---|---|---|
| Chronic ischemic heart disease: | ||||||
| MESAMI 2 | 90 | Autologous BM | Ischemic CM | Phase II | IM | NCT02462330 |
| HUC-Heart | 79 | Autologous/Allogeneic BM | Ischemic CM-Pre CABG | Phase I/II | IM | NCT02323477 |
| UCMSC-Heart | 40 | Allogeneic UC | Ischemic CM, HF | Phase I/II | IC | NCT02439541 |
| Dai et al. | 45 | Allogeneic UC | Ischemic CM | Phase I/II | Collagen Scaffold | NCT02635464 |
| TAC-HFT II | 55 | Autologous BM ± CSC | Ischemic CM | Phase I/II | Saline | NCT02503280 |
| SEESUPIHD | 6 | Allogeneic UC | Ischemic CM | Phase I/II | IC | NCT02666391 |
| TPAABPIHD | 200 | Autologous BM | Ischemic CM | Phase I/II | NYD | NCT02504437 |
| Guoping et al. | 10 | Allogeneic UC | Ischemic CM | Phase I | IM | NCT01946048 |
| WJ-ICMP Tria | 160 | Allogeneic WJ | Ischemic CM | Phase II | IC/IV | NCT02368587 |
| CONCERT-HF | 144 | Autologous BM + c-kit+ CSC | Ischemic CM | Phase II | IM | NCT02501811 |
| Kyriakos et al. | 5 | Allogeneic BM | Ischemic CM | Phase II/III | IM | NCT01759212 |
| Maskon et al. | 80 | Autologous BM | Ischemic dilated CM | Phase II | IC | NCT01720888 |
| STEM-VAD | 30 | Allogeneic BM | Ischemic CM | Phase II | IV | NCT03925324 |
| Scorem-Cells | 40 | Allogeneic WJ | Ischemic CM | Phase I/II | IC | NCT04011059 |
| Harjula et al. | 60 | Autologous BM | Ischemic CM+CABG | Phase II | IM | NCT0041818 |
| TEAM-AMI | 124 | Autologous BM | Ischemic CM | Phase II | IC | NCT0304772 |
| Acute Myocardial infarction: | ||||||
| Musialek et al. | 115 | Allogeneic BM(Cardiocell) | Acute MI | Phase II/III | IC | NCT03418233 |
| Lien et al. | 8 | Allogeneic UC | Acute MI | Phase I | IC/IV | NCT04056819 |
| PT Prodia | 15 | Allogeneic UC | Acute MI | Phase I/II | IC/IV | NCT04340609 |
| ESTIMATION | 50 | Autologous BM | Acute MI | Phase III | IM | NCT01394432 |
| CIRCULATE | 115 | Allogeneic WJ | Acute MI | Phase II/III | IC | NCT03404063 |
| RELIEF | 135 | Autologous BM | Acute MI | Phase III | IC | NCT01652209 |
| AMICI | 105 | Allogeneic BM | Acute MI | Phase II | IC | NCT01781390 |
| PUMP1 | 60 | Allogeneic BM (Provacel) | Acute MI | Phase I | IV | NCT00114452 |
| Prochymal | 220 | Allogeneic BM | Acute MI | Phase II | IV | NCT00877903 |
[i] Abbreviations: BM-Bone marrow; WJ-Wharton Jelly; UC-Umbilical Cord; CM-Cardiomyopathy; MI-Myocardial Infarction; TESI- Transendocardial stem injection; IM-intramyocardial; IC-intracoronary; IV-intravenous; NYD-Not Yet determined; MSC-Mesenchymal stem cells; CABG-Coronary artery bypass grafting; CSC-Cardiac stem cells.
