Indolent non-Hodgkin’s lymphomas (NHLs), also referred to as mature B-cell neoplasms, are a diverse category of malignancies that have comparable disease progression and therapeutic approaches. Even though these lymphomas are generally regarded as incurable, most patients can expect to live as long as people their age, with the exception of those who are young at the time of diagnosis and those who, after receiving initial systemic treatment, progress quickly or develop into aggressive lymphomas.[1] Indolent lymphomas are characterized by a chronic relapsing-remitting disease course, with patients usually exposed to several successive treatment courses. Indolent lymphomas include follicular lymphoma, marginal zone lymphoma, lymphoplasmacytic lymphoma, small cell lymphocytic lymphoma/chronic lymphocytic leukemia, prolymphocytic leukemia, and hairy cell leukemia.[2] Early treatment does not increase survival, and many patients can be watched for years before treatment is necessary, according to a key tenet of managing indolent NHLs. Although antibody-based therapy has greatly improved prognosis, there is no cure for the disease. In asymptomatic patients, waiting is still a viable choice. Maintaining the highest possible quality of life has been the aim of the treatment.
Patients with symptomatic advanced indolent NHL and mantle cell lymphoma (MCL) are now receiving the usual initial treatment with immunochemotherapy regimens based on Rituximab. Patients with chronic lymphocytic leukemia and those whose indolent NHL advanced during or within 6 months of receiving rituximab or a rituximab-containing regimen have shown clinical activity with bendamustine, an alkylating agent. The combination of bendamustine plus rituximab (BR) was also shown to be active in patients with advanced indolent NHL.[3] For the initial management of individuals with indolent lymphoma, BR continues to be the preferred regimen. This use is based on the preliminary findings from the BRIGHT trial and the StiL NHL 1 study.[4]
In patients with previously untreated indolent NHL or MCL, Rummel et al.’s data demonstrate that BR is more beneficial and less harmful than R-CHOP.[5] Patients treated with BR had a significantly longer progression-free survival (PFS) than those treated with R-CHOP after a median follow-up of 45 months (69.5 months vs. 31.2 months).[6]
Although the therapeutic superiority of the BR regimen has been proven in large-scale clinical trials, there is a growing body of research looking into the real-world outcomes of this regimen. Furthermore, there is a paucity of data when it comes to the efficacy of this regimen in lower-middle-income countries, where factors such as high drug costs, particularly of Rituximab, may affect compliance with therapy and hence outcomes. Therefore, the purpose of this study is to carry out a retrospective analysis of real-world data from a tertiary care hospital in Pakistan, a lower-middle-income country, and to make a thorough inquiry into the clinical efficacy of BR regimen for B-cell indolent NHLs in terms of response rate and survival.
A retrospective study was conducted from January 2015 to July 2022 among patients with indolent B-cell lymphomas treated with bendamustine in combination with Rituximab at our institute, Aga Khan University Hospital, Karachi, Pakistan. The patient list was extracted from the electronic database. All patients underwent pre-BR assessments, including physical examination, routine hematology, and biochemistry testing, as well as positron emission tomography (PET)/computed tomography (CT) imaging before therapy. In addition to this, basic demographic parameters were also extracted from the electronic database. All patients under the age of 18 years and those with a diagnosis of transformed lymphoma, discordant histology, or any other hematological disorder were excluded from the study. Patient selection and follow-up are summarized in Figure 1.
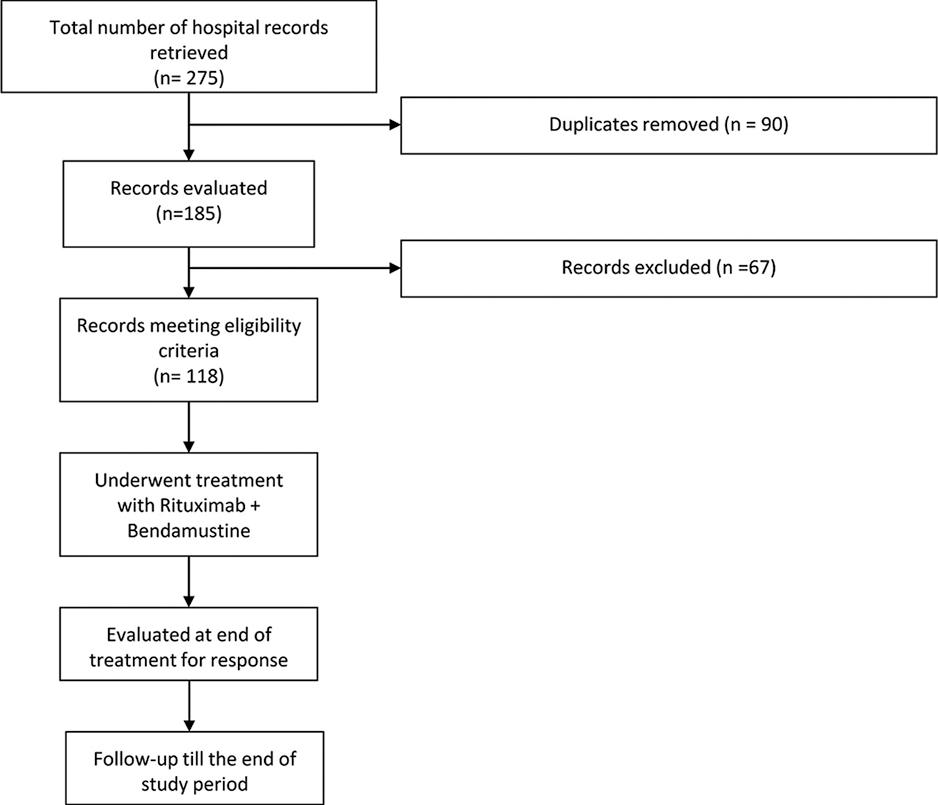
Flowchart summarizing patient selection and follow-up
The study received approval from the Ethical Review Committee (ERC) of Aga Khan University (ERC #: 2023-8787-25313) and the College of Physicians and Surgeons of Pakistan. Data collection was conducted through the electronic medical records of eligible patients, ensuring patient confidentiality. Information pertaining to demographics, disease characteristics, Type of indolent lymphoma, and clinical outcomes after receiving Rituximab-Bendamustine were recorded in a standardized pro forma.
All patients received the following treatment regimen: Bendamustine 90 mg/m2 and Rituximab 375 mg/m2 with a 4-week (28-day) cycle for 6 cycles. Patients thereafter did not receive rituximab maintenance.
Response to treatment was assessed every three to four cycles with a PET scan or CT scan (clinician preference) and then with PET/CT at the end of treatment. Complete metabolic response (CMR) was defined as negative findings on a PET scan performed after the completion of systemic chemotherapy. The following PET findings were considered to be consistent with CMR: nodal or extranodal sites with Deauville scores of 1, 2 or 3 with or without an extranodal mass. On the other hand, the following findings were considered indicative of a partial metabolic response: Deauville scores of 4 or 5 with decreased uptake compared to baseline and the presence of residual masses. Those patients whose disease returned after achieving complete remission were considered to have relapsed. The refractory disease was taken to be any case where patients did not respond to treatment or progressed during systemic treatment.
The PFS was taken to be the length of the time for which the patient remained alive with disease but without its progression or relapse after remission. In addition, the overall survival (OS) was calculated as the length of time for which the patient remained alive after the start of treatment. Initiation of treatment was taken as the starting point for both PFS and OS since many patients may not have disease progression for a long time and do not require treatment straightaway.
All statistical analyses were performed in Stata version 16 (from StataCorp). Baseline demographics and diagnostic data were summarized as a mean with standard deviation for continuous variables and as a percentage frequency for categorical variables. The percentage of all patients experiencing a CMR or refractory disease was calculated. In addition, the percentage of patients who initially had a CMR but then relapsed was also calculated.
To quantify the PFS and OS, a survival analysis was conducted. The results were summarized as the median PFS or OS with its 95% confidence interval and were depicted using a Kaplan–Meier survival function curve. Where the Kaplan–Meier function curve did not allow us to calculate a median value, the restricted mean and lower-quartile values were reported instead. In addition, 2-year and 5-year survival rates were also tabulated.
We also conducted a subgroup analysis for PFS and OS, stratifying by malignancy type and stage. A logrank test was conducted to compare between the various subgroups. In addition, we also conducted a Cox regression to compare the effect of these factors on PFS and OS, taking demographic factors such as age and pre-existing comorbidities as moderating factors. A P < 0.05 was taken as the cutoff for significance for all statistical analyses.
Our study consisted of 118 patients, with n = 76 males and n = 42 females. The patients in the sample were mostly of an older age, with the mean age standing at 62 years (standard deviation: 9.8 years). Hypertension was the most common comorbid in our patient sample, affecting 54.2% of patients (n = 64), followed by diabetes mellitus in 38% (n = 45) and ischemic heart disease in 22 patients.
The follow-up rate was 76.3% (n = 90) at 2 years, 42.4% (n = 50) at 3 years, 29.7% (n = 35) at 4 years, and 22.0% (n = 26) at 5 years. The median follow-up time was 29 months.
The most common histopathology at the time of diagnosis was follicular lymphoma in n = 61 and chronic lymphocytic leukemia in n = 29 patients, accounting for over 76% of the patient sample [Table 1]. In n = 66 (56%), the disease presentation was stage IV at the time of diagnosis, and 31% (n = 36) presented with stage III disease. Only 12% (n = 14) of patients presented with stage I or II disease.
Characteristics of study cohort (n=118) with indolent Non‑Hodgkin lymphoma
| Variable | Statistic (%) |
|---|---|
| Histopathology | |
| Follicular lymphoma | 61 (51.6) |
| Marginal zone lymphoma | 5 (4.2) |
| Small cell/chronic lymphocytic lymphoma | 29 (24.5) |
| Lymphoplasmacytic lymphoma | 3 (2.5) |
| Hairy cell leukemia | 1 (0.8) |
| Mantle cell lymphoma | 10 (8.5) |
| B-cell lymphoproliferative disorder | 9 (7.6) |
| Stage | |
| I | 1 (0.8) |
| II | 13 (11) |
| III | 36 (30.5) |
| IV | 66 (55.9) |
| Data not available | 2 (1.6) |
| Primary Treatment Outcome | |
| Complete metabolic response | 86 (72.8) |
| Partial metabolic response | 21 (17.7) |
| Refractory | 8 (6.7) |
| Data not available | 3 (2.5) |
After primary treatment with the BR regimen, 73% (n = 86) experienced a CMR. Partial response was seen in 17.7% (n = 21) of patients and 7% of patients had refractory disease. However, of those who underwent a CMR, 31.40% (n = 27) ended up experiencing a relapse.
At the time of data collection, 15% of patients had expired. Survival analysis revealed the restricted mean OS [Figure 1] to be 140 months (95% CI: 120–160). The lower quartile for OS stood at 50 months, while the 2-year OS was 91.8% (95% CI: 84.8–95.7), and the 5-year OS was 71.0% (95% CI: 53.7–82.7). Through survival analysis, we were also able to calculate the median PFS to be 80 months (95% CI: 43-N/A), with a lower-quartile value of 26 months [Figure 2]. In addition, the 2- and 5-year PFS rates stood at 76.1% (95% CI: 66.5–83.4) and 59.7% (95% CI: 45.2–71.4), respectively.
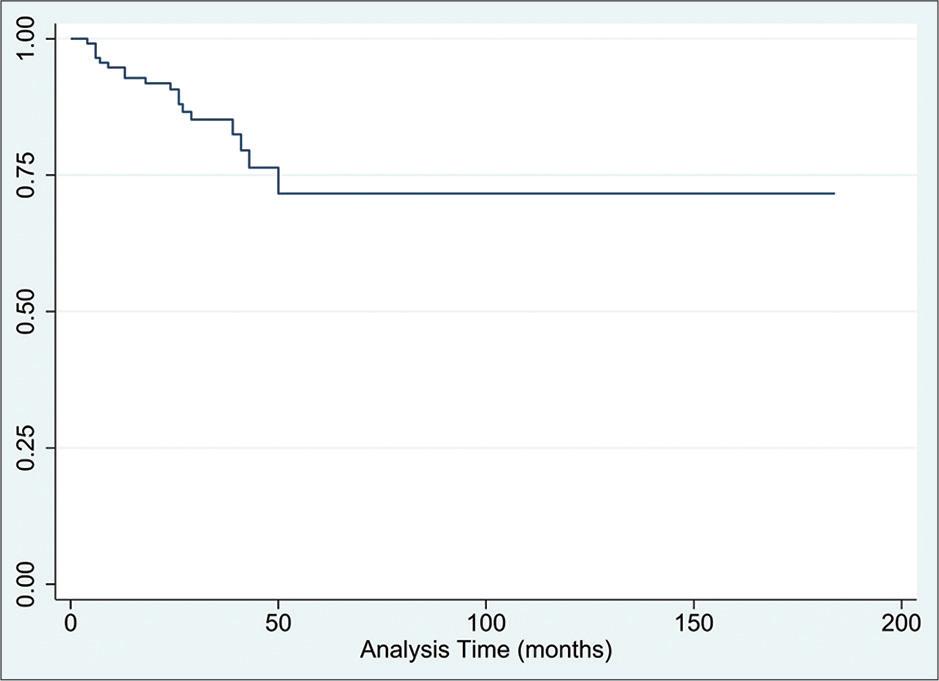
Overall survival following the treatment combination of Rituximab-Bendamustine in patients diagnosed with indolent lymphomas
The results of our subgroup analysis, where we stratified by histopathological type and disease stage, are summarized in Tables 2 and 3. Table 2 represents the subgroup analysis for 2-year and 5-year OS rates. The histopathological subtypes with the highest 2-year and 5-year OS rates were follicular and marginal zone, with these rates being 94.8% (95% CI: 84.7–98.3) and 74.5% (95% CI: 50.3–88.1), respectively, for follicular. Meanwhile, both the 2-year and 5-year values for marginal zone lymphomas were 100%. However, none of the histopathological subtypes had OS rates that differed significantly from the overall cohort. In addition, the stage of disease was found not to have a significant bearing on the OS rate (P = 0.13).
Subgroup analysis for 2-year and 5-year overall survival rates
| Strata | Overall survival rate (95% CI) | Log-rank P-value | |
|---|---|---|---|
| 2-year (%) | 5-year (%) | ||
| Histopathology | |||
| Follicular | 94.8 (84.7–98.3) | 74.5 (50.3–88.1) | 0.30 |
| Marginal zone | 100 (N/A) | 100 (N/A) | 0.31 |
| Small cell/chronic lymphocytic | 84.3 (63.4–93.8) | 52.3 (19.2–77.6) | 0.054 |
| Lymphoplasmacytic | 66.7 (5.4–94.5) | - | 0.57 |
| Hairy cell | 100% (N/A) | - | 0.77 |
| Mantle cell | 90.0 (47.3–98.5) | - | 0.73 |
| B-cell lymphoproliferative disorder | 100 (N/A) | - | 0.89 |
| Stage | |||
| I | 100 (N/A) | 100 (N/A) | 0.13 |
| II | 63.6 (29.3–84.6) | 63.6 (29.3–84.6) | |
| III | 100 (N/A) | 63.7 (26.9–85.7) | |
| IV | 91.9 (81.6–96.6) | 78.0 (57.6–89.4) | |
The results for the subgroup analysis of 2-year and 5-year PFS rates are summarized in Table 3. The lowest 2-year and 5-year PFS rates were represented by the follicular subtype, at 63.8% (95% CI: 49.5–75.0) and 46.8% (95% CI: 28.4–63.1), respectively, which was found to be significantly lower than other subtypes (P = 0.002). On the other hand, the small cell subtype was the only subtype to have significantly higher PFS rates (P = 0.014), with a 2-year rate of 95.0% (95% CI: 69.5–99.3) and 5-year rate of 77.7% (95% CI: 28.2–95.1). Moreover, the stage of disease was found to significantly affect the PFS rate (P = 0.00).
Subgroup analysis for 2-year and 5-year progression-free survival rates
| Strata | Progression-free survival rate (95% CI) | Log-rank P-value | |
|---|---|---|---|
| 2-year (%) | 5-year (%) | ||
| Histopathology | |||
| Follicular | 63.8 (49.5–75.0) | 46.8 (28.4–63.1) | 0.002* |
| Marginal zone | 100 (N/A) | - | 0.40 |
| Small cell/chronic lymphocytic | 95.0 (69.5–99.3) | 77.7 (28.2–95.1) | 0.014* |
| Lymphoplasmacytic | 100 (N/A) | - | 0.30 |
| Hairy cell | 0.00 (N/A) | - | 0.00* |
| Mantle cell | 80.0 (40.9–94.6) | - | 0.59 |
| B-cell lymphoproliferative disorder | 100 (N/A) | - | 0.73 |
| Stage | |||
| I | 0.0 (N/A) | 0.0 (N/A) | 0.00* |
| II | 100 (N/A) | 100 (N/A) | |
| III | 72.5 (53.7–84.6) | 54.1 (29.2–73.6) | |
| IV | 75.9 (62.6–85.0) | 57.5 (37.0–73.5) | |
Note: indicates significant P values (< 0.05)
Cox regression revealed that age and preexisting comorbidities did not affect OS or PFS. In addition, none of the histopathological subtypes had a bearing on OS or PFS, with the exception of the hairy-cell subtype, which had a significant impact on PFS (P = 0.01) but not on OS (P = 1.00). The results for the Cox regression have been summarized in Table 4.
Cox-regression for factors affecting overall survival and progression-free survival
| Variable | Overall survival | Progression-free survival | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age | 1.03 (0.97–1.08) | 0.34 | 0.97 (0.94–1.01) | 0.18 |
| Comorbid | ||||
| Diabetes mellitus | 0.68 (0.24–1.88) | 0.45 | 0.81 (0.35–1.88) | 0.63 |
| Hypertension | 1.39 (0.45–4.32) | 0.57 | 1.10 (0.46–2.61) | 0.83 |
| Ischemic heart disease | 0.96 (0.26–3.45) | 0.94 | 0.76 (0.27–2.13) | 0.60 |
| Histopathology | ||||
| Follicular | 1.21 (0.15–9.86) | 0.86 | 2.03 (0.47–8.69) | 0.34 |
| Marginal zone | 6.48e-16 (0-N/A) | 1.00 | 0.45 (0.04–5.47) | 0.53 |
| Small cell/chronic lymphocytic | 3.15 (0.37–27.13) | 0.30 | 0.25 (0.03–1.94) | 0.19 |
| Lymphoplasmacytic | 2.43 (0.12–49.40) | 0.56 | 1.01e-15 (N/A-N/A) | |
| Hairy cell | 9.88e-15 (0-N/A) | 1.00 | 116.97 (6.44–2123.80) | 0.001* |
| Mantle cell | 1.00 (N/A) | N/A | 1.00 (N/A) | N/A |
| B-cell lymphoproliferative disorder | 1.60 (0.96–26.74) | 1.00 (0.14–7.37) | 0.998 | |
| Stage | 0.95 (0.49–1.87) | 0.89 | 0.67 (0.40–1.20) | 0.18 |
Note: indicates significant P values (< 0.05)
We conducted a retrospective analysis to investigate clinical outcomes in patients of indolent NHL undergoing primary therapy with a combination of Rituximab and Bendamustine, otherwise known as an R-Benda regimen. We examined the clinical outcomes in terms of the response rate, rate of relapse after complete remission, OS, and PFS in 118 patients undergoing treatment at the study institute over a period of 7½ years. The mean age of our sample was 62 years, which was consistent with the fact that, overall, NHLs mostly present between 65 and 74 years.[7] The prevalence of the mentioned comorbidities in our sample was comparable to that of the general Pakistani population.[8]
Most patients in our sample had follicular lymphoma (52%) on histopathology, followed by chronic lymphocytic lymphoma (24.58%). This was similar to other studies in the Pakistani population, which found follicular lymphoma and chronic lymphocytic lymphoma to be the two most common forms of indolent NHL.[9–11] Most of our patients presented with stage III (30.51%) or IV (55.93%) disease, with stage IV alone accounting for over half of the cases. The late stage of the disease at diagnosis is typical of both follicular lymphoma[12] and chronic lymphocytic lymphoma. This is well explained by the much milder symptomology of earlier-stage disease and the relative lack of B symptoms.[12,13]
Most patients in our study showed a CMR to the R-Benda combination, with 73% of patients achieving CMR. This was much higher than the 31% CMR rate of R-Benda reported by the BRIGHT study.[3] The complete response rate of our study was also considerably higher than the response rates reported by multiple studies evaluating real-world outcomes, hovering between 50% and 60%.[14–17] In particular, two studies from other South Asian settings, both involving patients with follicular lymphoma, by Paikaray et al.[18] and Gogia et al.,[19] reported lower complete response rates of 60% and 64.7%, respectively. However, a study by Castelli et al. reported an even higher rate of 86% in a sample of patients with splenic marginal zone lymphomas.[16] It would, therefore, seem that the exact range for the complete response rate might vary with the variety of indolent lymphoma and other factors, and the higher response rate reported by our study might be a result of the casemix of indolent lymphomas analyzed in our study as well as patient and population-level factors that were not controlled for.
Our survival analysis revealed the mean OS to be 140 months (95% CI: 120–160), with the lower quartile for OS being 50 months. We were unable to calculate a median value for OS due to the low number of deaths occurring in the sample and the shorter period of observation. These results, however, illustrate that for almost all patients, the OS was 50 months or longer despite the fact that most of the patients in our study presented with the late-stage disease at the time of diagnosis. This finding is not surprising, given the mild clinical course of indolent lymphomas, with many patients living to a normal life expectancy and dying of other causes.[1,13]
The median PFS in our study was 80 months (95% CI: 43-N/A), with the lower quartile for PFS being 26 months, indicating that almost all patients had a PFS of 26 months or longer [Figure 3]. This was comparable to findings from other studies on real-world experiences of Rituximab-Bendamustine therapy. For instance, Bond et al. found the PFS to be 39.5 months with the most ideal dosing.[14] In addition, Morigi et al. estimated the 6-year PFS rate to be 71.8%,[17] while the results of Gentile et al. showed the 2-year PFS rate to be 69.9%.[15] This was similar to the 76.17% (95% CI: 66.5–83.4) PFS rate in our study. It is worth noting that Morigi et al. found that most cases of relapse occurred within 2 years of the end of treatment,[17] indicating that in patients with a good initial response, the long-term prognosis was extremely favorable.
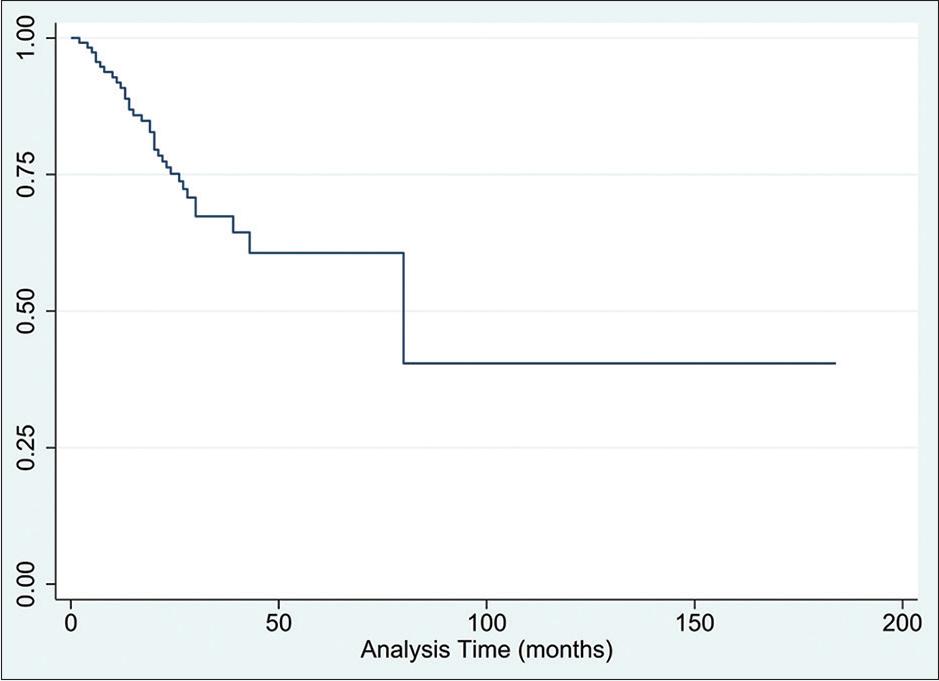
Progression free survival following the treatment combination of Rituximab-Bendamustine in patients diagnosed with indolent lymphomas
In our subgroup analysis, follicular lymphomas were found to have a significantly lower overall PFS rate. However, MCL is the subtype that is traditionally considered to carry a poorer prognosis.[5] The reason our study may have been unable to replicate this finding regarding MCLs may be the fact that there were only 10 cases of this histopathological type in our sample, hence, our analysis was not powered enough to detect a difference.
The limitations of our study included the fact that the reports from scans conducted outside our institute were not available in the hospital’s electronic database, thus limiting the number of patients that could be included in our analysis. Any data not available in the electronic database was inaccessible to us. Moreover, the observation time was very low for a considerable number of patients. In particular, the follow-up rate beyond the 2-year mark was low. Moreover, the retrospective design of our study might have been a source of bias in the study results. Additionally, the low number of cases of histopathological subtypes other than follicular and small cell lymphoma made it difficult to draw conclusions about these other subtypes.
The median OS in our study was 9.5 years with a PFS of 80 months with a BR regimen for indolent NHL. Overall, the prognosis for patients who experience a good initial response to treatment remains favorable and is comparable to the results with other treatment regimens. In addition, the results reaffirm findings from other studies showing the efficacy of such regimens in lower-middle-income settings. Further studies on real-world experiences from various settings will help solidify the results in favor of Rituximab-Bendamustine combination therapy. Such studies should also focus on the costing aspects of such treatments, especially in lower-middle-income settings, where drug costs can affect compliance with treatment and, hence, outcomes.