The prognosis of cancer patients has significantly improved with the success of chemotherapy and targeted therapies. However, anticancer medications may also have undesirable cardiovascular side effects, which can trigger cardiac dysfunction immediately or over time.[1] The second most common long-term cause of death among cancer patients is cardiovascular disease (CVD).[2] The complex molecular mechanisms by which some anticancer medications, in particular alkylating agents and anthracyclines, affect the structure and function of the heart have been uncovered by research in this field.[1] Inhibiting vascular endothelial growth factor and platelet-derived growth factor receptor causes a decrease in pro-survival signaling and angiogenesis, as well as impacts nitric oxide and calcineurin/nuclear factor of activated T cells. This results in hypertension, cardiomyocyte apoptosis, cardiac and mitochondrial dysfunction, and oxidative stress.[3] It has been found that damage to the heart in cancer patients undergoing chemotherapy and immune checkpoint inhibitors is worsened by interleukin-1β. Before chemotherapy, cardioprotective agents can prevent chemotherapy-related cardiotoxicity and heart failure.[4] Improving cardiac care requires identifying patients who are at high risk for cardiovascular problems brought on by cancer therapies and implementing early diagnosis, prevention, and treatment measures.[5]
Despite various studies discussing the cardiotoxic potential of anticancer drugs, few have discussed potential preventive measures that could be taken to prevent the cardiotoxic potential of anticancer drugs. The previous systematic review of this topic included studies up to 2020; therefore, there is a need to summarize the findings of the newly published literature. Hence, we analyzed studies published between 2021 and 2023 to provide the latest and revised version of the literature. This systematic review evaluated the effectiveness of physical and pharmaceutical interventions for cancer therapy-induced cardiotoxicity. Cancer treatment using drugs such as anthracyclines, monoclonal antibodies, and tyrosine kinase inhibitors is effective in treating cancer patients; however, the cardiotoxic side effects of these drugs are inevitable. Prophylactic and treatment approaches are urgently needed to prevent and alleviate these side effects. This systematic review focused on the cardioprotective effects of drugs such as angiotensin-converting enzyme inhibitors, beta-blockers, angiotensin receptor blockers, sodium-glucose cotransporter-2 inhibitors, and physical therapy. The outcomes of this review may provide a reference for prophylaxis and treatment and may help reduce the incidence of CVDs caused by cancer treatment.
This study aimed to examine the current knowledge on cardiotoxicity caused by anticancer medications and examine physiological and pharmacological techniques to avoid or reduce these negative effects. The objective was to aid in the creation of safer, more efficient, and safer cancer treatment plans for cancer patients.
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines as it is a widely recognized framework for conducting systematic reviews in health care. By adhering to the PRISMA guidelines, we ensured transparency and reliability in our research methodology.[6]
We thoroughly searched multiple databases, including PubMed/Medline, Google Scholar, Wiley, Embase, and Scopus. The keywords string that we utilized were “Cardiotoxicity” OR “Cardiotoxic effects” AND “anticancer drugs” OR “antineoplastic agents.”
We followed the population, intervention, comparator, and outcome criteria to frame the selection of studies. Clinical trials and observational cohort studies describing interventions that prevent the cardiotoxicity of anticancer drugs in cancer patients aged >18 years were included in our systematic review. Studies published in English between 2021 and 2023 with the full text available were selected by the authors. Studies in other languages with full texts unavailable or published before 2021 were excluded. All laboratory studies, including those involving animal subjects, were removed during screening. The details of the inclusion and exclusion criteria are listed in Table 1.
Studies inclusion and exclusion criteria
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Cancer patients of age>18 years receiving chemotherapy | Participants under the age of 18 years Patients not receiving anticancer therapy Non-human subjects |
| Interventions | Any pharmacological or physiological intervention preventing chemotherapy-induced cardiotoxicity | Interventions other than that prevent cardiotoxicity |
| Comparator | Any | Not specified |
| Outcomes | Not specified | Not specified |
| Study designs | Clinical trials (randomized, non-randomized, blinded, open-label, and in any phase) Observational cohort studies Any interventional study | Study designs other than that listed in inclusion criteria. E.g., case reports, case series, reviews (narrative, scoping, systematic), cross-sectionals, short communication (letters, commentaries), book chapters, study protocols, and conference papers. |
| Year of publication | Studies published between 2021-2023 | Studies published before 2021 |
| Language | English Language | Languages other than English |
| Country | No restriction | No restriction |
| Full text | Full text available | Full text not available |
After searching the studies using data sources, two authors independently screened the articles based on the inclusion and exclusion criteria, and if any controversy appeared, it was sorted out by panel discussion and involving a third person. After removing duplicates, we retrieved titles and abstracts of the remaining articles, including relevant studies, and excluded those that were irrelevant or ineligible. Finally, full-text screening of the papers was performed to assess qualifying studies. If the reviewers found any ambiguity or controversy during the screening process and study eligibility could not be sorted with mutual agreement, a third person was allowed to settle.
Data extraction was performed using the Microsoft Excel software. Study characteristics, such as first author, year of publication, study design, interventions, and outcome measures, were extracted. Moreover, we extracted the participants’ characteristics, such as the total sample size, age, and sex. The data are summarized in Table 2.
Tabulation of extracted data
| Sr. No. | Lead author | Study design | Participants | Interventions | Outcome measures | Results |
|---|---|---|---|---|---|---|
| 1. | D.J. Kerrigan (2023) | RCT | Total subjects=29
| Control group: No intervention.
| Primary outcome
| Cardiac Rehabilitation by exercise training can improve CRF in cancer patients. |
| 2. | M. Lee (2021) | RCT | Total subjects=195
| Experimental group Candesartan or carvedilol Control group No intervention | Primary outcome
| The protective effect of candesartan on DISC and changes in LVEF was greater than that of carvedilol and that of the control group. |
| 3. | Postigo-Martin (2021) | ATOPE Trial | Total subjects=150 Feasibility phase
| Multimodal therapeutic exercise (aerobic, strength, motor control exercises, myofascial techniques, and breathing exercises) | Main outcome
| Exercise has a significant positive effect on attenuation of treatment-related cardiotoxicity. |
| 4. | Wihandono (2021) | RCT | Total subjects=74
| Experimental group: Lisinopril and bisoprolol Control group: No intervention | Primary outcome Changes in LVEF
| A significant positive effect of Lisinopril and bisoprolol against cardiotoxicity by causing a decrease in LVEF |
| 5. | Carlos A (2022) | Cohort | Total subjects=128
| Case patients SGLT2 inhibitors with anthracycline therapy | Primary cardiac outcome
| SGLT2 inhibitors can prevent cardiac events due to adverse effects of anthracycline treatment. Moreover, they are safe to use in patients receiving chemotherapy. |
CRF: Cardiorespiratory fitness, CV: Cardiovascular, DISC: Doxorubicin-induced subclinical cardiotoxicity, LVEF: Left ventricular ejection fraction, LA: Left atrium, LV: Left ventricle, HF: Heart failure, EF: Ejection fraction, SGLT-2: Sodium-glucose cotransporter-2
We used the Critical Appraisal Skills Program (CASP) checklist for quality assessment of the studies.[7] Two separate checklists were used in the clinical trials and observational cohort studies. The clinical trial checklist has 11 questions in four sections that assess the validity and quality of the basic study design, study methodology, reporting of results, and implications of results. The cohort checklist has 12 questions in three sections that analyze the validity and implications of the study results. The authors who did data extraction also performed a quality assessment of the studies using the CASP checklist. The checklist questions were answered by authors individually and when there was any ambiguity, it was sorted out by a panel decision. All the studies were of good quality. The quality assessment results are reported in Tables 3 and 4.
Critical appraisal of clinical trials
| Appraisal Criteria | Yes | No | Can’t Tell |
|---|---|---|---|
| SECTION-A: Is the basic study design valid for an RCT? | |||
| 1. Did the study address a clearly focused research question? | 4 | ||
| 2. Was the assignment of participants to interventions randomized? | 4 | ||
| 3. Were all participants who entered the study accounted for at its conclusion? | 1 | 3 | |
| SECTION-B: Was the study methodologically sound? | |||
| 4. Questions about blinding. | |||
| •Were the participants ‘blind’ to the intervention they were given? | 4 | ||
| •Were the investigators ‘blind’ to the intervention they were giving to participants? | 4 | ||
| •Were the people assessing/analyzing outcome/s ‘blinded’? | 4 | ||
| 5. Were the study groups similar at the start of the randomized controlled trial? | 4 | ||
| 6. Apart from the experimental intervention, did each study group receive the same level of care (that is, were they treated equally)? | 4 | ||
| SECTION-C: What are the results? | |||
| 7. Were the effects of intervention reported comprehensively? | 4 | ||
| 8. Was the precision of the estimate of the intervention or treatment effect reported? | 4 | ||
| 9. Do the benefits of the experimental intervention outweigh the harms and costs? | 2 | 2 | |
| SECTION-D: Will the results help locally? | |||
| 10. Can the results be applied to your local population/in your context? | 4 | ||
| 11. Would the experimental intervention provide greater value to the people in your care than any of the existing interventions? | 1 | 1 | 2 |
Critical appraisal of an observational cohort study
| Appraisal criteria | Yes | No | Can’t Tell |
|---|---|---|---|
| SECTION-A: Are the results of the study valid? | |||
| 1. Did the study address a clearly focused issue? | ✓ | ||
| 2. Was the cohort recruited in an acceptable way? | ✓ | ||
| 3. Was the exposure accurately measured to minimize bias? | ✓ | ||
| 4. Was the outcome accurately measured to minimize bias? | ✓ | ||
| 5. (a) Have the authors identified all important confounding factors? | ✓ | ||
| 5. (b) Have they taken account of the confounding factors in the design and/or analysis? | ✓ | ||
| 6. (a) Was the follow-up of subjects complete enough? | ✓ | ||
| 6. (b) Was the follow-up of subjects long enough? | ✓ | ||
| SECTION-B: What are the results? | |||
| 7. Have they reported the rate or the proportion between the exposed/unexposed, ratio/rate difference? | ✓ | ||
| 8. Are the results precise? | ✓ | ||
| 9. Do you believe the results? | ✓ | ||
| SECTION-C: Will the results help locally? | |||
| 10. Can the results be applied to the local population? | ✓ | ||
| 11. Do the results of this study fit with other available evidence? | ✓ | ||
| 12. Was the study suitable to imply for practice? | ✓ |
A total of 1367 articles were identified through a literature search of various databases. After removing 34 duplicate and irrelevant studies, 405 articles were screened. Of these, 164 were sorted for abstract retrieval after removing articles published in languages other than English and those with irrelevant study designs. Of these, 22 were assessed based on the full text. Finally, five studies were finalized to add to our systematic review, of which three were randomized controlled trials (RCTs), one was an ATOPE trial, and one was a retrospective cohort study. The literature search results are presented in the PRISMA flow diagram [Figure 1].
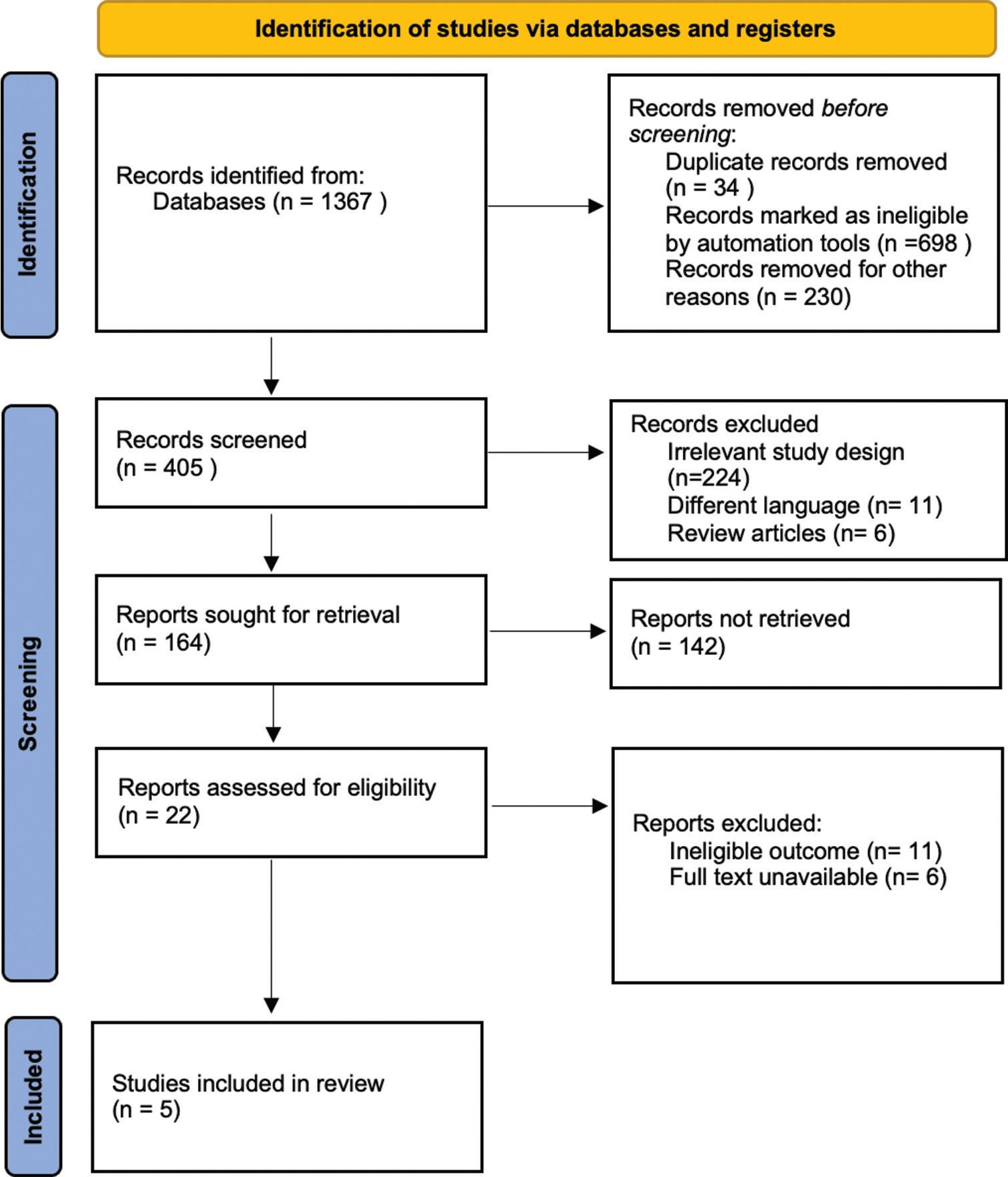
Preferred Reporting Items for Systematic Reviews and Meta-analysis flow chart
In total, 448 patients were included in this study. All patients who participated in the trials were female patients with breast cancer receiving chemotherapy. In this cohort study, the patient population included both males and females suffering from cancer and diabetes mellitus. In the experimental group, one study adopted a combination therapy involving lisinopril and bisoprolol. One study used carvedilol and candesartan therapy. Two studies adopted exercise interventions, and one study involved monotherapy with an SGLT-2 inhibitor, empagliflozin, canagliflozin, or dapagliflozin. None of the included studies involved interventions in the control group. Primary outcomes assessed in the analysis were left ventricular ejection fraction (LVEF), LV strain, cardiomyopathy, and heart failure (HF). The overall study and participant characteristics are presented in Table 2.
CASP checklists for RCTs and cohort studies were used. The authors performed a quality assessment of selected studies while extracting data from the articles. Each author independently answered the checklist questions, and if there was any confusion or ambiguity, it was dealt with authors’ panel discussion. All trials had a clearly defined research question, and randomization of the participants was carried out. As far as the quality of a cohort study is concerned, it has valid and precise results according to the CASP checklist for cohort studies. All the studies were of good quality. The results of the quality assessment of the studies are reported in Tables 3 and 4.
The primary and secondary outcomes of the selected studies are given in Table 2. Two studies investigated the cardioprotective role of exercise in patients undergoing chemotherapy. One study reported the use of a combination therapy involving lisinopril and bisoprolol. Lisinopril and bisoprolol therapy were started 24 h before the first cycle of chemotherapy. One study supported the prophylactic effects of carvedilol and candesartan on adverse cardiac events resulting from chemotherapy. Candesartan (4mg q.d) and carvedilol (3.125 mg q.d) were started simultaneously in two different groups of patients who were scheduled for doxorubicin chemotherapy. Finally, one study described the role of SGLT-2 inhibitors empagliflozin, canagliflozin, and dapagliflozin in lowering cardiac events in patients during chemotherapy. All pharmacological interventions and exercise have positive effects on mitigating the cardiotoxicity associated with anticancer drugs.
In the ATOPE trial, exercise intervention had a significant positive effect on LVEF changes and, hence, on the prevention of cardiotoxicity in patients with breast cancer.[8] Moreover, cardiac rehabilitation significantly improved cardiorespiratory fitness in the experimental group compared with the control group (P = 0.009).[9]
Candesartan substantially lowered the incidence of early DOX-induced subclinical cardiotoxicity (DISC) compared to the control group with no intervention (P = 0.022). The incidence of early DISC after the administration of candesartan was 18.6% in the control group and 4.9% in the treatment group. Compared with the control group, carvedilol significantly reduced the decrease in LVEF (P < 0.001).[10]
The mean change in LVEF was –0.27 ± 5.73 in the treatment arm and –5.52 ± 8.90 in the control arm, and a potential difference (P = 0.017) was observed in LVEF changes after six chemotherapy cycles between the treatment and the control group, which shows a significantly good effect of the combination therapy on LVEF.[11]
SGLT-2 inhibitors such as empagliflozin, canagliflozin, and dapagliflozin were used in the treatment group. A significant reduction in cardiac events was observed in patients receiving either of these medications compared with the control group (3% vs. 20%, P = 0.025). Moreover, overall mortality was also lower in the intervention group (9% vs. 43%; P < 0.001). Hence, SGLT-2 inhibitors are effective and safe for mitigating cardiotoxicity in cancer patients.[12]
Our comprehensive systematic review provided critical insights from a careful examination of five selected studies, comprising three RCTs, one ATOPE trial, and one retrospective cohort study. These findings highlight the multifaceted landscape of cardiotoxicity mechanisms and the potential effectiveness of various intervention strategies. Exercise interventions and combination therapy with lisinopril and bisoprolol have emerged as a promising avenue, showing a positive influence on LVEF improvement and mitigation of cardiotoxicity, particularly in patients with breast cancer. Therapies with candesartan and carvedilol have demonstrated potential in reducing early DISC. The inclusion of SGLT-2 inhibitors in a retrospective cohort study suggests the possibility of reducing cardiovascular events. These findings underscore the importance of individualized approaches for counteracting cardiotoxic effects in the context of anticancer treatment.
Despite the valuable insights gained, it is imperative to acknowledge the inherent limitations of the included studies. Variations in the study design, patient populations, and interventions across the selected trials introduced heterogeneity in our analysis. The small sample sizes and varying follow-up durations in some studies may constrain the generalizability of our findings. The number of studies included in our systematic review is small due to the restriction of inclusion criteria to only newly published literature from 2021 to 2023. In addition, the potential for publication bias should not be underestimated because studies with favorable outcomes tend to be more readily published, which may have affected the overall results. Recognizing these limitations serves as a guiding principle for future research endeavors to enhance the credibility and reliability of the evidence aimed at preventing cardiotoxic effects.
Our systematic review has gained strength from a meticulous and methodical approach to synthesizing the existing literature concerning the mitigation of cardiotoxic effects in anticancer therapy. Adherence to the PRISMA guidelines ensures transparency and rigor in every phase of the review process. However, it is essential to acknowledge these potential weaknesses. The relatively limited number of selected studies stemming from our stringent inclusion criteria may have contributed to the lack of diversity in the evidence pool. In addition, while our intent was to include only high-quality studies, variations in study design and quality levels among the chosen trials may have influenced the overall robustness of the evidence. Future systematic reviews in this field could benefit from a more extensive selection of studies and stricter quality assessments.
Comparing our findings to existing systematic reviews and guidelines in the field, we found alignment and extension. Tranchita et al. emphasized the importance of exercise interventions, which correspond with our own results, highlighting exercise as a beneficial strategy for cardioprotection.[13] Similarly, Cruz et al. conducted a review that, while acknowledging the significance of exercise, also explored the efficacy of pharmacological interventions, particularly ACE inhibitors, as promising approaches for mitigating cardiotoxicity in cancer patients.[14] Our research complements these findings by further investigating the effectiveness of candesartan and carvedilol in reducing early cardiotoxicity. In addition, our study introduces a novel perspective on using SGLT-2 inhibitors, a facet not previously covered by the European Society of Cardiology Guidelines on Cardio-oncology.[15] These comparisons underscore the evolving landscape of cardiotoxicity prevention strategies and the contribution of our study to this dynamic field.
In conclusion, our systematic review underscores the intricate nature of combatting cardiotoxicity and highlights the potential for intervention strategies. Exercise interventions and pharmacological agents such as candesartan and carvedilol, combination therapies, and SGLT-2 inhibitors all exhibit promise for preserving cardiac function and reducing cardiotoxicity. Acknowledging the limitations of the included studies and variations in interventions underscores the need for careful interpretation. These findings have substantial implications for contemporary clinical practice, emphasizing the need for tailored approaches to cardioprotection in patients with cancer. Furthermore, they delineated a promising avenue for future research, underlining the significance of larger, more homogeneous study populations and extended follow-up periods to unravel the enduring impact of interventions and their repercussions on long-term cardiovascular outcomes. Ultimately, our systematic review advances our understanding of the effective prevention of cardiotoxic effects associated with anticancer drugs, thereby enhancing the quality of care for patients in this challenging therapeutic context.
In conclusion, the present systematic review supports the significant positive effects of exercise and the above-mentioned pharmacological interventions in preventing and mitigating cardiotoxic events due to anticancer drugs such as anthracyclines, monoclonal antibodies, and tyrosine kinase inhibitors. These interventions should be implemented in oncological practice to provide better care and safety for patients with cancer.