Squamous cell carcinoma (SCC) is the most common of head-and-neck cancers.[9,14] It is treated by surgery alone or in combination with radiotherapy or chemotherapy, depending on the stage. Post-tumor surgery, ablative defect varies in dimensions and compromise esthetics and function. The reconstruction of defects can vary from primary closure to healing by secondary intention, local flaps, skin grafts, regional flaps, or microvascular reconstruction.[7,24] Microvascular free flaps remain the gold standard but require a dedicated team, increased operation room time, and hence, an enhanced cost both in terms of resources and workforce. Medically compromised patients are good candidates for reconstruction with a submental (SM) flap. Previously, the pectoralis major myocutaneous pedicled flap was the workhorse flap for the reconstruction of head-and-neck defects. The disadvantages include increased bulk, difficulty in flap design in female patients, and morbidity in patients with restrictive lung disease. Although first described by Martin et al. in 1993, it was not until 1996 that Sterne et al. used this flap in intraoral defect reconstruction.[20,26] It is a reliable flap, with good color match and proximity to the oral cavity with less donor site morbidity in an expert hand.[19] The biggest concern is the potential risk of harboring cervical metastatic disease at the donor site.[1,5] Some still question the oncological safety of the SM flap and recommend that an alternate reconstructive option should be used if there are suspicious lymph nodes (LNs) in level I.[21] Others have suggested that SM flaps can be harvested even in the presence of pathological neck nodes, and this can be achieved with careful and vigilant neck dissection.[10,25] Our study aims to investigate the oncological safety of a SM flap when utilized as a reconstructive option for head-and-neck tumors.
All the patients were retrospectively analyzed from the data retrieved from the electronic health information system of Shaukat Khanum Cancer Hospital and Research Center, Lahore, Pakistan, from 2015 to 2021. The tumors were staged according to the American Joint Committee on Cancer 8th edition guidelines.[3] This study examined patients of all ages who had biopsy-confirmed oral cavity SCC and were treated with surgical intervention followed by reconstruction utilizing an SM flap. We excluded all the subjects who had a histopathological diagnosis other than SCC and those who had a history of previous treatment in the head-and-neck region by surgical or non-surgical modalities. Data collected included the gender, tumor subsite, clinical and pathological stage, nodal yield, local or regional recurrence, survival rates, and complications. The institutional review board of Shaukat Khanum Memorial Cancer Hospital and Research Center, Lahore, Pakistan, approved the study.
The SM artery is a pedicled flap based on the terminal branch of the facial artery and has a diameter of approximately 1.7 mm. Between one and four perforators supply the cutaneous component of the flap and anastomose with the contralateral SM artery. Flap dimensions of approximately 7–8 cm in width and 15–18 cm in length are possible depending on skin laxity.[4,13] The length of the vascular pedicle is around 8 cm, and the average diameter of the SM artery is 2.5 mm.[8,11] The “skin pinch” test confirms the skin laxity to assess the maximum flap dimensions available with primary closure. The flap has a wide arc of rotation and can reach pre-auricular, cheek, and intraoral defects. The flap drains through the accompanying SM vein and eventually into the external or internal jugular veins.[18] We use the same technique as previously described in our published study.[12] We mark the flap dimensions according to the defect size. An elliptical incision is marked, and the superior incision is marked just under the inferior border of the mandible from one angle of the mandible to the other angle of the mandible. The lower incision is marked according to the size of the defect. We first raise the lower neck flap in a subplatysmal plane to the clavicle. The upper neck flap is raised carefully to prevent damage to the marginal mandibular nerve. Facial and SM vessels are identified following exposure to the submandibular gland. The anterior belly of the digastric muscle is exposed and raised with the flap. At this point, the underlying platysma muscle is sutured with the overlying skin paddle to prevent shearing of the cutaneous perforators. Next, we move to the contralateral side, and the subplatysmal neck flap is raised to the midline. While using a proximally based flap, we ligate the facial vessel above the origin of the SM artery.
Careful dissection is done through the submandibular gland to identify and tie the glandular branches while preserving the SM vascular pedicle. In our experience, the venous drainage pattern is very variable and confers a degree of difficulty in flap harvesting. The flap typically drains into the facial vein and the internal jugular vein. At times, the flap drains directly into the jugular venous system through the anterior division of the retromandibular vein. Therefore, preserving all veins going into the flap is critical until the venous drainage channel has been reliably identified. The flap is temporarily sutured to facial skin while performing neck dissection to prevent unnecessary movement jeopardizing the skin perforators. Depending on the defect site, the flap can then be transported medial or lateral to the mandible. The reach of the flap can be improved by ligating the facial artery cephalad to the origin of the SM branch; in this way, the flap has only anterograde flow. Another way to improve the length of the vascular pedicle and, hence, the flap is to ligate the facial artery more proximally inside the gland; this can give an additional reach of 1–2 cm.[23] Before the final inset, we check the distal edge of the flap for adequate perfusion and reassess the pedicle for any torsion compromising vascularity.
A total of 88 oral cavity SCC subjects (71 males and 17 females) with a mean age of 55.3 years (range: 25–79 years) were retrieved from the database. No known risk factors have been identified in 29 (33.3%) patients, while 59 (66.6%) patients had one or more risk factors. The risk factors identified were cigarette smoking (13.3%), smokeless tobacco chewing (15.5%), betel nut usage (35.4%), and alcohol use (1.1%). Buccal mucosa was the most commonly involved subsite (42%) followed by lower alveolus (30.7%) and (27.3%) tongue. The follow-up period ranged from 4 to 75 months, with a mean of 33.5 months. The SM flap reconstruction was performed on all 88 subjects. Of those, 3 subjects had complete loss of the flap, 17 subjects had incomplete loss/partial necrosis where the flap was salvaged with debridement, and 68 subjects had an uneventful recovery of their flaps. Clinical and demographic data are shown in Table 1.
Characteristics of demographics and clinical data (n=88)
| Variable | Number of sub-jects (percentage) |
|---|---|
| Gender | |
| Male | 71 (80.68) |
| Female | 17 (19.31) |
| Median age | 55.3 (25–79) years |
| Mean follow-up months | 33.5 (4–75) months |
| Risk factors: (Smoking, chewable tobacco, betel nut, alcohol) | |
| Yes | 59 (67.04) |
| No | 29 (32.95) |
| Primary site | |
| Buccal mucosa | 37 (42.05) |
| Tongue | 24 (27.27) |
| Lower alveolus | 27 (30.68) |
| pTNM stage: | |
| No primary tumor (salvage surgery post-induction chemotherapy) | 3 (3.40) |
| Stage I | 14 (15.90) |
| Stage II | 27 (30.68) |
| Stage III | 18 (20.45) |
| Stage IV | 26 (29.54) |
| Flap complications | |
| Complete loss | 3 (3.40) |
| Partial loss/salvaged | 17 (19.31) |
| Uneventful recovery | 68 (77.27) |
Three-fourths of the subjects had no lymph node involvement on the final histopathology report. The complete pattern of cervical lymph node metastasis is summarized in Table 2.
Breakdown of cervical lymph node involvement (n=88)
| Positive cervical lymph node (involvement by levels) | Number of subjects (percentage) |
|---|---|
| No lymph node involved | 66 (75.0) |
| Ipsilateral positive level I | 11 (12.5) |
| Ipsilateral positive levels I and II | 2 (2.3) |
| Ipsilateral positive level I and III | 1 (1.1) |
| Bilateral positive level I | 1 (1.1) |
| Ipsilateral positive level II | 7 (8.0) |
There was no recurrence in approximately 58% of the subjects. A total of 16 subjects (18.1%) presented with a demonstrable local recurrence in the flap. We included the subjects with local recurrences, which we could not ascertain clearly as originating within or away from the flap. The number of subjects labeled as having a local recurrence in the flap bed was 4 (4.5%) after excluding subjects with positive/close surgical margins and positive level I LNs. An analysis of the incidence of local recurrences over varying timelines, namely 6, 12, and 24 months, suggested that 2 subjects had local disease recurrence in a SM flap within 6 months, 1 subject within 12 months, and 1 subject after 49 months. The pattern of recurrence is shown in Table 3.
Summary of pattern of recurrence (n=88)
| Recurrence | Number of subjects (percentage) |
|---|---|
| No recurrence | 51 (58.0) |
| Local recurrence | 10 (11.4) |
| Local recurrence away from flap | 10 (11.4) |
| Local and nodal recurrence | 6 (6.8) |
| Nodal recurrence | 9 (10.2) |
| Distant metastasis | 1 (1.1) |
| Lost to follow-up | 1 (1.1) |
Furthermore, among 16 subjects with primary local recurrence in the final histopathology report, factors such as close margin, involvement of disease at margins, and positive LN at the level I were present in the flap. These were taken into consideration, as shown in Table 4. The results showed that 4 subjects had a local recurrence despite having clear surgical margins and no positive pathological node at level I.
Details of subjects with primary local recurrence (n=26)
| Total patients with recurrence | Number of patients (percentage) |
|---|---|
| Involved margins | 7 (26.9) |
| Close margins | 5 (19.2) |
| Level I positive lymph node | 5 (19.2) |
| Involved/close margins and level I positive lymph node | 5 (19.2) |
| Clear margins and level I (pN0) | 4 (15.3) |
N0: No cervical lymphadenopathy at the level I in the final histopathological report
This study investigated the oncological safety of a SM flap as a reconstructive option in head-and-neck cancer surgery. After excluding all the subjects with suspected cervical lymph node metastasis, we only had 4.5% of subjects in which the local recurrence was attributable to a positive cervical lymph node from the donor bed. The goal of cancer surgery is to excise the tumor with safe margins; this leaves a defect that has cosmetic as well as functional consequences. The advent of microvascular surgery has revolutionized the reconstruction of such defects. There are some situations in which microvascular surgery cannot be performed, such as patients with medical co-morbid conditions who are unable to tolerate prolonged general anesthesia. Microvascular surgery procedures require specialized units with dedicated teams and intensive care units for post-operative monitoring. The limitation of resources puts a considerable burden on low-income countries. There was a need to look for a local flap as a good alternative to the tissue lost in terms of volume and function. The SM flap was described by Martin et al.[20] in 1993 and later was used to reconstruct an oral cavity defect by Sterne et al.[26] Despite early skepticism about its oncological safety, the flap is now widely utilized in head-and-neck reconstruction. The SM flap has many advantages, including a long pedicle and a good arc of rotation. The flap can also be used in the reverse pattern with pedicles between 4 × 6 cm and 4 × 8 cm in size.[15] Given the skin color and texture similarity, the SM flap is an ideal replacement for facial skin if used to cover extra oral defects. The resultant flap defect can be closed primarily, which can be ensured pre-operatively by doing “the pinch test”.[1,17] The flap does not require a secondary procedure for flap division. During flap harvesting, including the anterior belly of the digastric muscle is advisable due to many advantages, including the increase in bulk of the flap, protection of the vascular pedicle, and increase in blood supply by muscle inclusion. Mylohyoid muscle can also be included in the flap to protect the perforators; this maneuver also increases venous return. However, including the mylohyoid muscle can compromise the pedicle length. There is also a risk of damage to the marginal mandibular nerve.[18,28] None of the participants in our study had any permanent deficit of the marginal mandibular nerve. A disadvantage of flap is hair growth when used intraorally. Most subjects had undergone post-operative radiotherapy and had no problems with hair growth. Others were advised laser hair removal.
The most notable concern against using a SM flap for reconstruction in the setting of head-and-neck tumors is the possible transfer of nodal disease to the reconstruction site. The primary drainage basins of most oral cavity tumors are the SM and submandibular LNs. There have been concerns about the continuity of neck dissection and tumor seeding when isolating the pedicle of the SM flap. According to Amin et al.,[2] there is a minimal chance of cancer recurrence when the dissection plane is subplatysmal. They also concluded that recurrent disease was more related to the disease potential of the tumor rather than the flap itself. Authors have demonstrated that the chance of recurrent disease is negligible if anatomic planes are respected.[1,2,5] We always do the lymph node dissection before raising the flap; thus, we can abandon the SM flap if there are suspicious LNs in level I. The use of a frozen section is also a helpful adjunct, but the flap should be abandoned if there is a suspicion of lymph node involvement, which was not present on the pre-operative scan. Multinu et al. have demonstrated the flap’s oncological safety and recommended that it should be only used in patients with no clinical and radiological preoperative cervical lymph node involvement.[21] The study by Sittitrai et al. demonstrated reconstruction in 35 patients and found that there is no difference between the rate of primary recurrence of patients with involved and clear level I LNs[25] (P = 0.64, 95% CI 0.24–10.49) this corresponds to our result (fig 2). All the primary recurrences were outside the flap reconstructed area. A few studies have sought to investigate the oncological safety of a SM flap in the presence of involved LNs at level I. The largest series comes from Ninth Peoples Hospital, Shanghai.[27] The authors operated on 160 patients, and they compared the results of pN0 and pN+ T1-T2 tumors. They concluded that there was no difference in disease-free survival and recurrence pattern in both groups and that a SM flap was a safe option if meticulous dissection was performed. This result is very similar to our results (fig 1). We are cautious in following these recommendations and have not performed SM flap reconstruction in any patient with suspicious lymphadenopathy at level I.
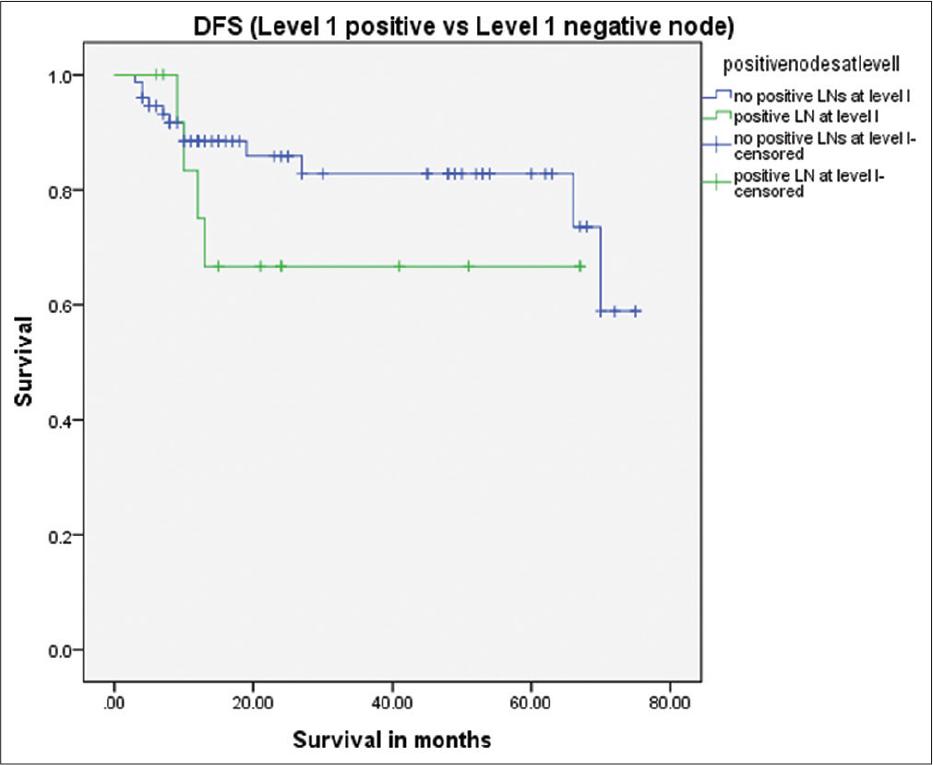
Survival difference between level I positive versus level I negative patients (P = 0.27)
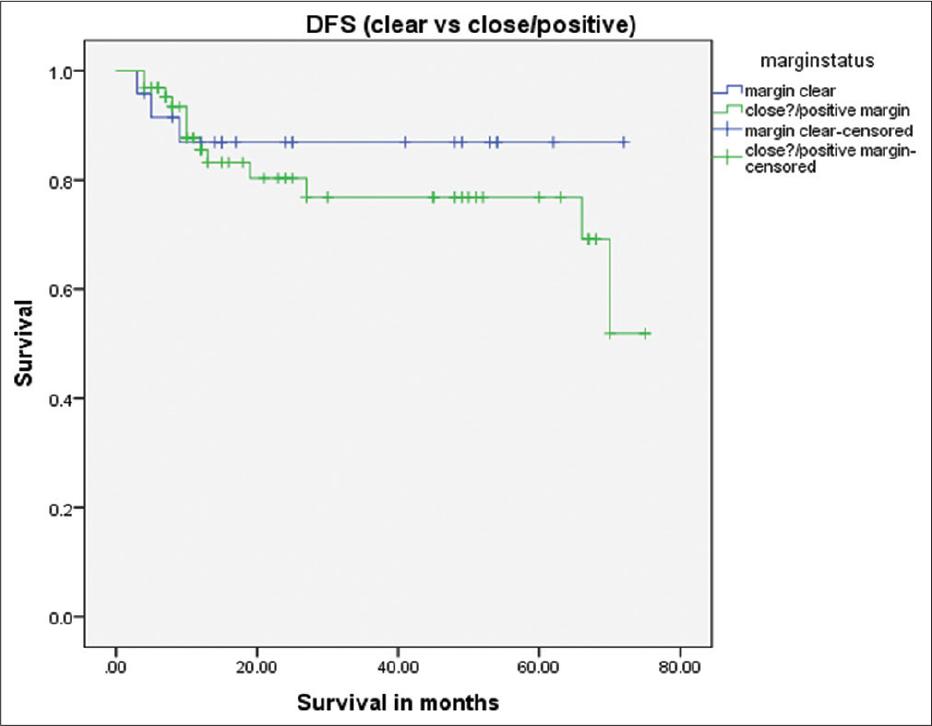
Survival difference between clear versus close/positive tumor margins P = 0.47
After excluding subjects with a primary recurrence in the presence of close/involved margins, we had just 4 (4.5%) subjects who had a recurrence in the SM flap area. There were 1, 1, 0, and 2 subjects in T stages I, II, III, and IV, respectively. The rate of primary recurrence in our study corresponds to the other studies.[2,6,16,22,23,27] Out of these 16 subjects, there were only 4 subjects who did not have a positive node at level I and clear surgical margins. The recurrence in these subjects at the primary site could be due to aggressive tumor characteristics. We do not have sufficient evidence regarding the time elapsed since surgery to label the primary recurrence as being due to the possible micrometastasis from the fibrofatty tissue transferred from the neck to the defect site with the flap. Sometimes, it is also difficult to tell if the recurrence had its epicenter in the flap or away from the flap, especially when the recurrence is very close to the flap or if there is a delay of a few weeks in patients’ follow-up. We encountered this difficulty in 3 of our subjects, and we considered these recurrences as originating from the flap. Cariati et al.[6] had a series of 9 patients, out of which 1 (11.1%) had a local recurrence. Their sample size was small, and the follow-up was 24 months. Saguillo et al. did SM flap reconstruction in 20 patients and reported local recurrence in only 1 (5%) patient.[23] This corresponds to the rate of recurrence of the present study. Saguillo et al. did not mention the follow-up or comment on the surgical margin status. Pradhan et al. performed SM flap reconstruction in 30 patients of SCC of different sub-sites of the oral cavity.[22] They did not find local recurrence in operated cases, but their follow-up was only 6 months. Sittitrai et al. had a sample size of 35 patients with a mean follow-up of 23 (range: 11–48) months. They observed six recurrences, none of which originated from the SM flap.[25] Wang et al. performed the largest study up to date on SM flap reconstruction.[27] They operated on 160 patients with SM flaps with a local tumor recurrence rate of 4.37% (7 patients), which is very similar to our local recurrence rate. Their result differed because they only included T1-T2 SCCs; they operated on N+ necks, and all of their recurrences were outside the SM flap. As already mentioned, we found it very difficult to label some recurrences as arising from within the SM flap or away from the flap. This was due to the late reporting of some subjects who have come from a neighboring country or far-flung areas of our country. We had a mean follow-up of 33.5 months which is longer than all the studies mentioned above except the study of Wang et al., who had a mean follow-up of 46.9 (range: 2–108) months. A comparative study was performed by Kramer et al.,[16] comparing the recurrence rate of two groups, one treated with a radial forearm free flap and the other with an SM flap. The observed recurrence rate was 11% as compared to 4.5% in our cohort.
The SM flap is reliable, has a good color match, avoids additional cost as compared to a free flap reconstruction, and, most importantly, is oncologically safe. Good pre-operative planning and keeping a backup flap in case a SM flap cannot be safely done is a prerequisite for a successful surgical procedure.