Scheme 1
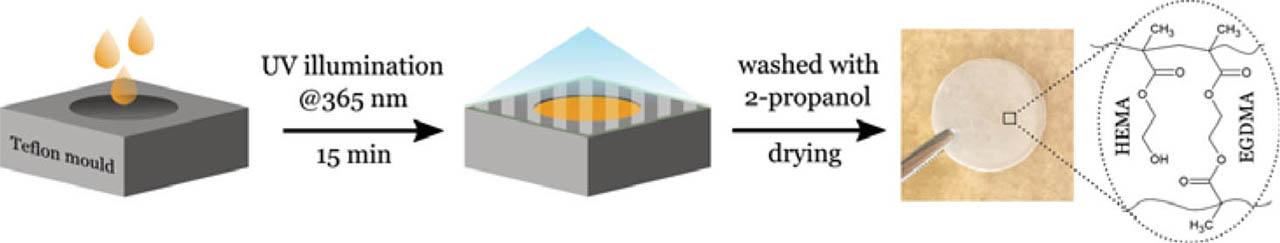
Fig. 1
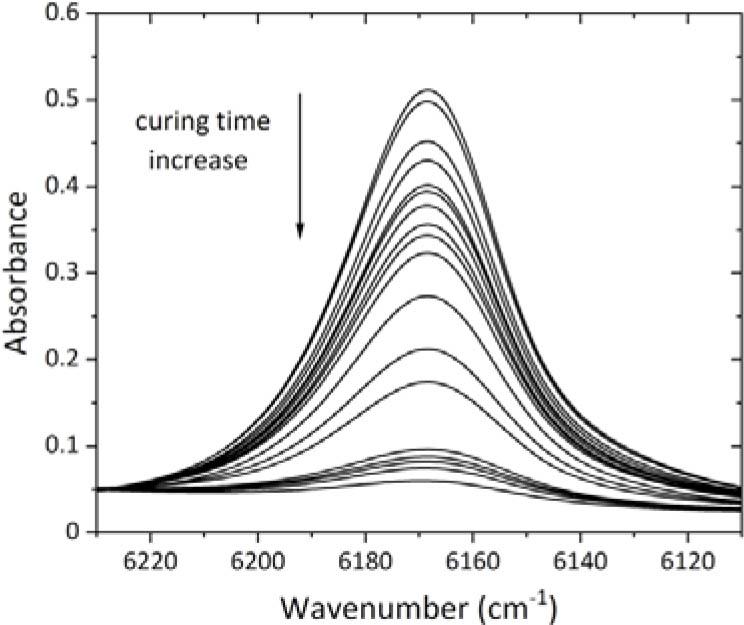
Fig. 2
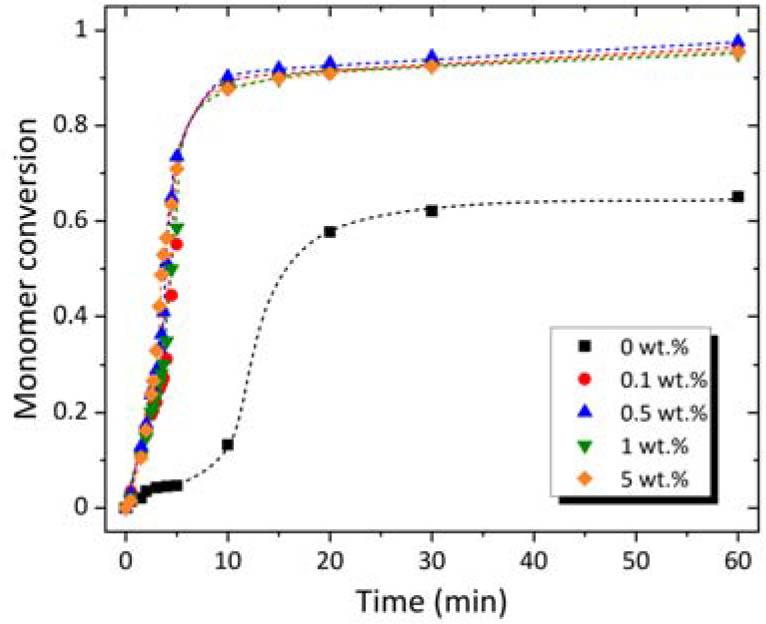
Fig. 3
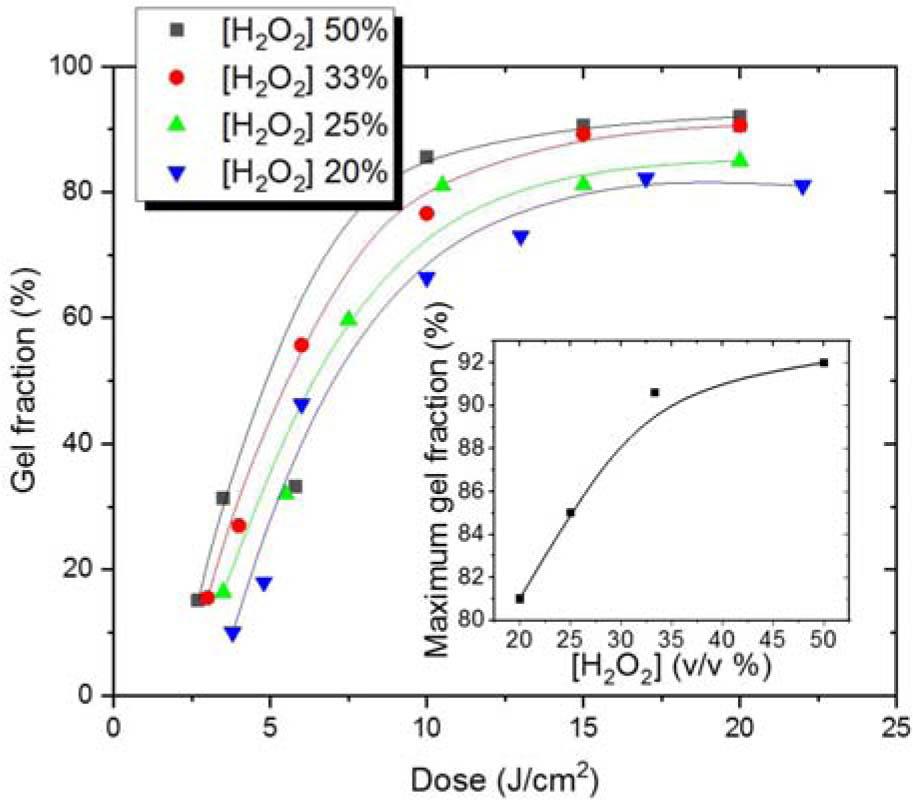
Fig. 4
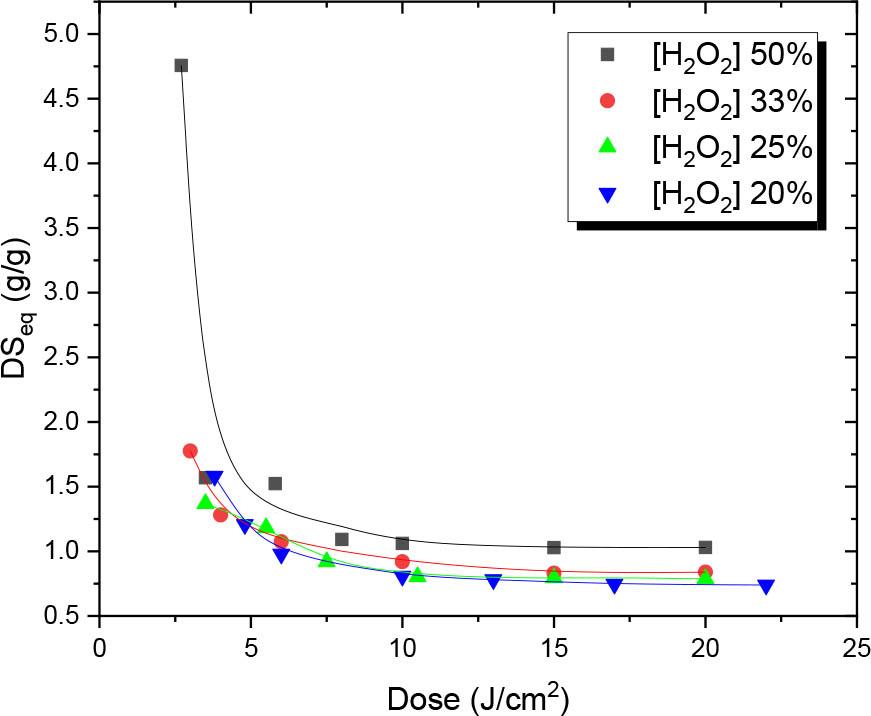
Fig. 5
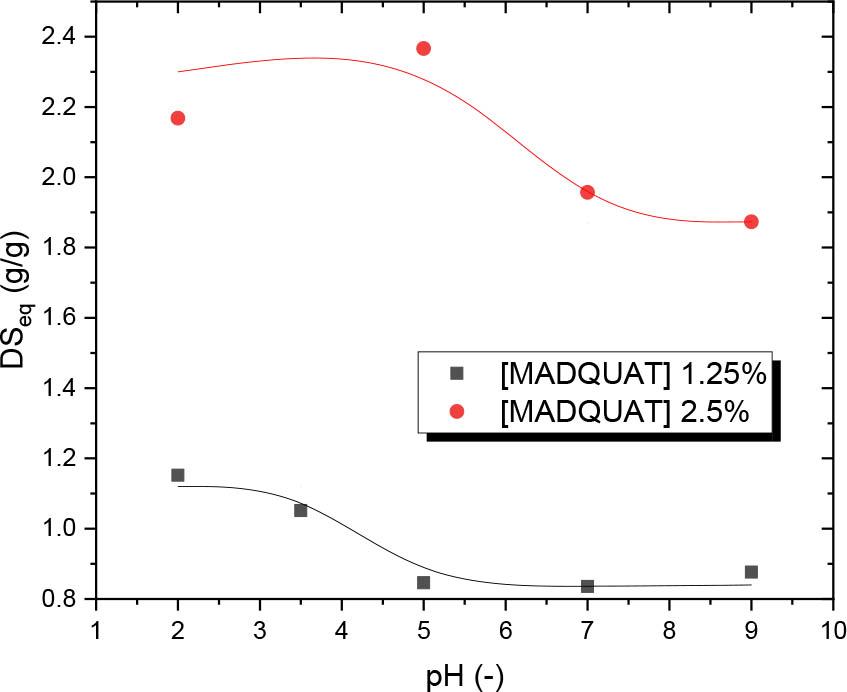
Fig. 6
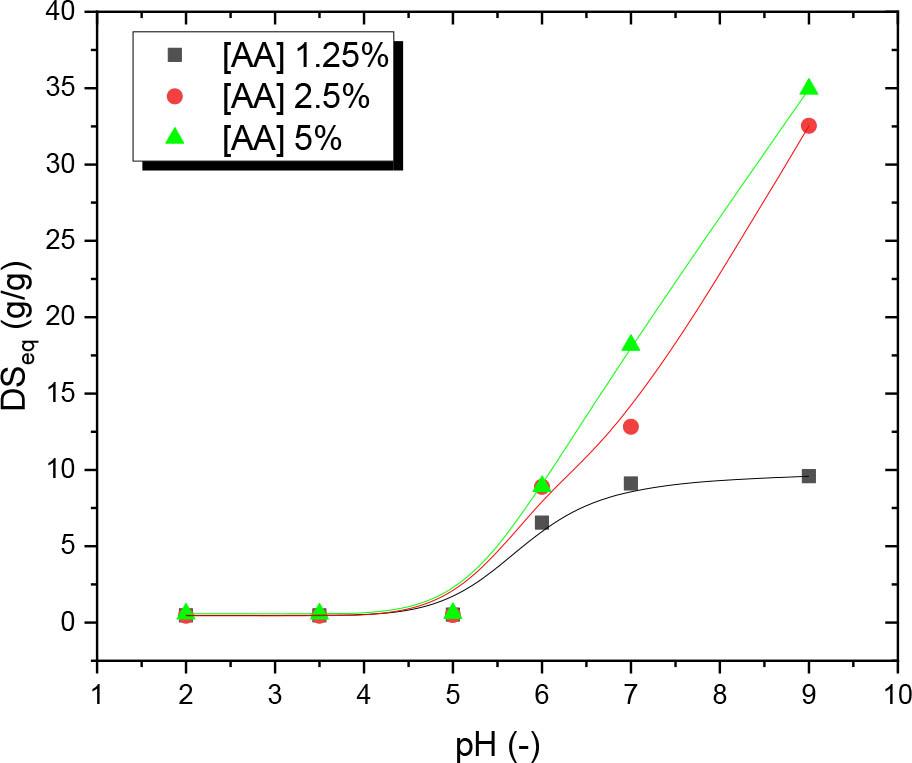
Fig. 7
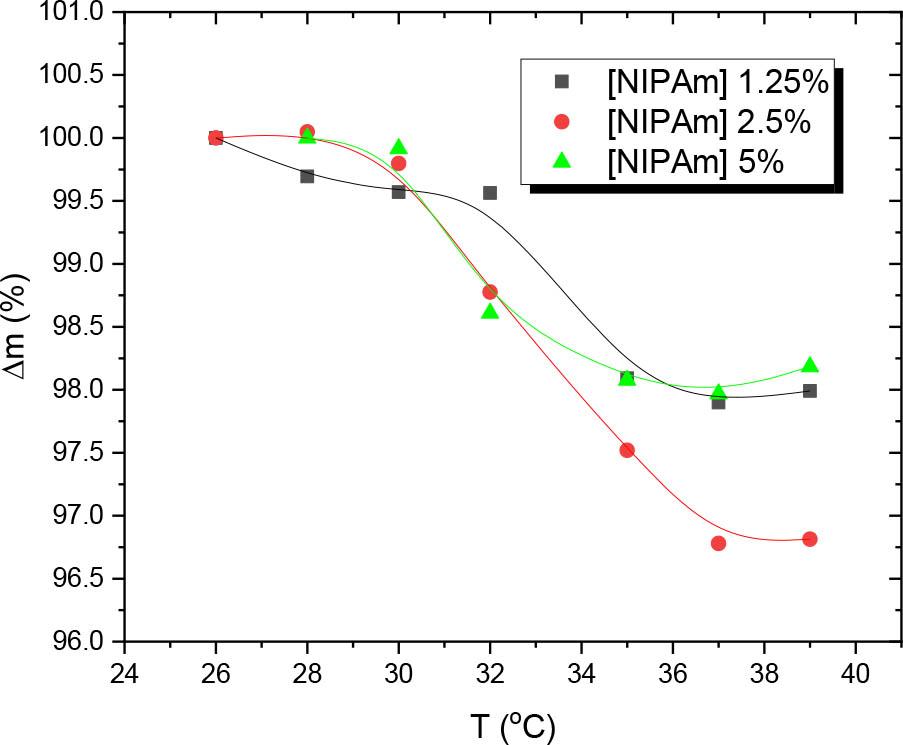
Fig. 8
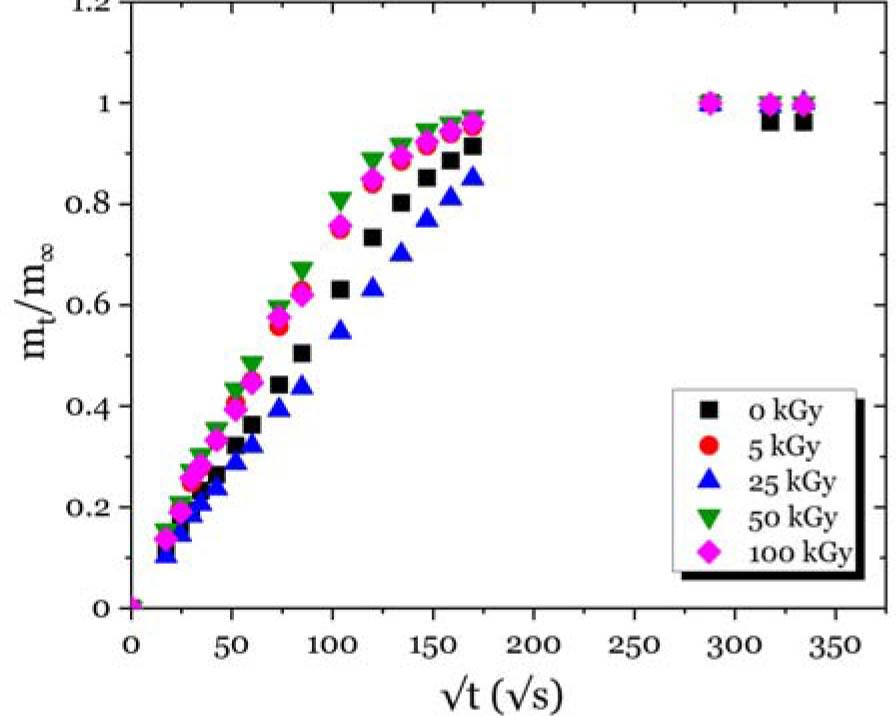
Fig. 9
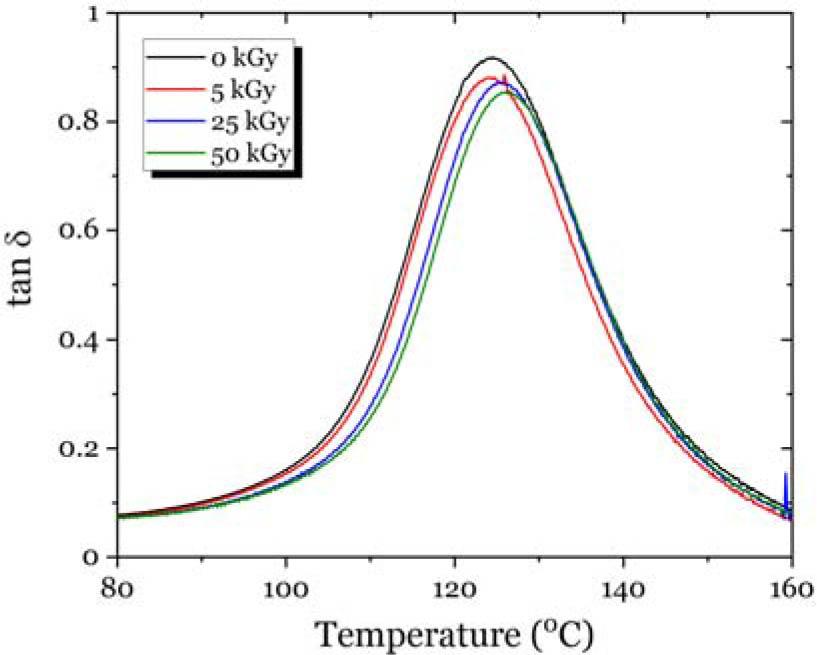
Fig. 10
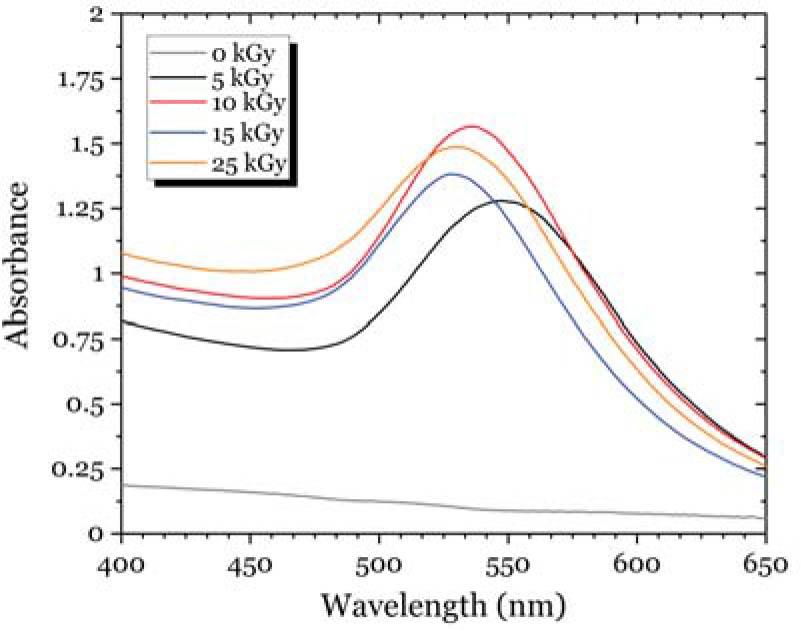
Fig. 11
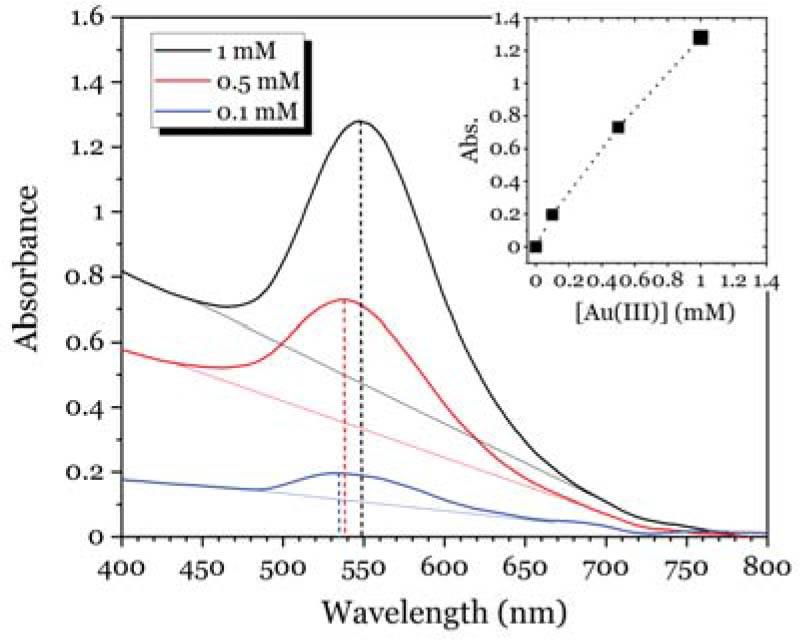
Fig. 12
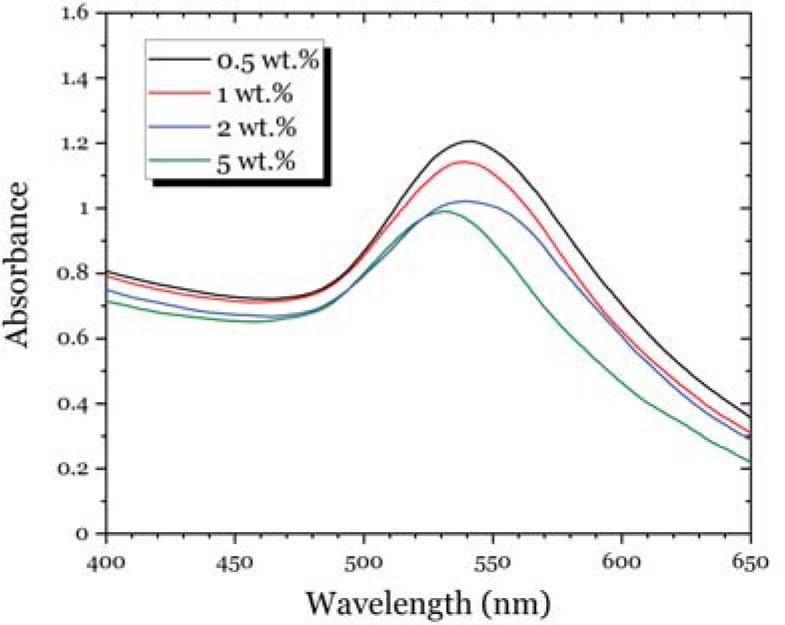
Fig. 13

Fig. 14

Gelation dose (Dg) and probability ratio of chain breakage to cross-linking (p0/q0) for hydrogels based on HEMA/EGDMA with the addition of a different amount of H2O2 (30% aqueous solution) synthesized by UV irradiation (254 nm, irradiance 2_6 mW·cm−2)
| Sample make-up | HEMA/EGDMA (99/1): 50 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 67 vol.% 30% H2O2: 33 vol.% | HEMA/EGDMA (99/1): 75 vol.% 30% H2O2: 25 vol.% | HEMA/EGDMA (99/1): 80 vol.% 30% H2O2: 20 vol.% |
|---|---|---|---|---|
| Sample composition | methacrylate functions 4.123 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 5.497 mol·L−1 H2O2 3.263 mol·L−1 | methacrylate functions 6.184 mol·L−1 H2O2 2.448 mol·L−1 | methacrylate functions 6.596 mol·L−1 H2O2 1.958 mol·L−1 |
| Dg (J·cm−2) | 2.33 | 2.55 | 2.92 | 3.55 |
| p0/q0 | 0 | 0 | 0 | 0.25 |
Gel fractions for hydrogels based on HEMA/EGDMA/H2O2 (30% aqueous solution) with various amounts of N-isopropylacrylamide, synthesized by UV irradiation (254 nm, irradiance 2_6 mW·cm−2, total exposure dose 8 J·cm−2)
| Sample make-up | HEMA/EGDMA (99/1): 50 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 48.75 vol.% NIPAm: 1.25 wt% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 47.5 vol.% NIPAm: 2.5 wt% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 45 vol.% NIPAm: 5 wt% 30% H2O2: 50 vol.% |
|---|---|---|---|---|
| Sample composition | methacrylate functions 4.123 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 4.020 mol·L−1 NIPAm 0.110 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 3.917 mol·L−1 NIPAm 0.221 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 3.710 mol·L−1 NIPAm 0.442 mol·L−1 H2O2 4.895 mol·L−1 |
| Gel fraction (%) | 82.0 | 75.8 | 70.3 | 65.7 |
Gel fractions for hydrogels based on HEMA/EGDMA/H2O2 (30% aqueous solution) with various amounts of AA, synthesized by UV irradiation (254 nm, irradiance 2_6 mW·cm−2, total exposure dose 8 J·cm−2)
| Sample make-up | HEMA/EGDMA (99/1): 50 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 48.75 vol.% AA: 1.25 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 47.5 vol.% AA: 2.5 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 45 vol.% AA: 5 vol.% 30% H2O2: 50 vol.% |
|---|---|---|---|---|
| Sample composition | methacrylate functions 4.123 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 4.020 mol·L−1 AA 0.182 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 3.917 mol·L−1 AA 0.364 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 3.710 mol·L−1 AA 0.729 mol·L−1 H2O2 4.895 mol·L−1 |
| Gel fraction (%) | 82.0 | 92.2 | 90.9 | 88.3 |
Gel fractions for hydrogels based on HEMA/EGDMA/H2O2 (30% aqueous solution) with various amounts of MADQUAT (75% aqueous solution), synthesized by UV irradiation (254 nm, irradiance 2_6 mW·cm−2, total exposure dose 8 J·cm−2
| Sample make-up | HEMA/EGDMA (99/1): 50 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 48.75 vol.% MADQUAT 1.25 vol.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 47.5 vol.% MADQUAT 2.5.% 30% H2O2: 50 vol.% | HEMA/EGDMA (99/1): 45 vol.% MADQUAT 5 vol.% 30% H2O2: 50 vol.% |
|---|---|---|---|---|
| Sample composition | methacrylate functions 4.123 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 4.020 mol·L−1 MADQUAT 0.049 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 3.917 mol·L−1 MADQUAT 0.097 mol·L−1 H2O2 4.895 mol·L−1 | methacrylate functions 3.710 mol·L−1 MADQUAT 0.194 mol·L−1 H2O2 4.895 mol·L−1 |
| Gel fraction (%) | 82.0 | 75.1 | 65.3 | 50.9 |