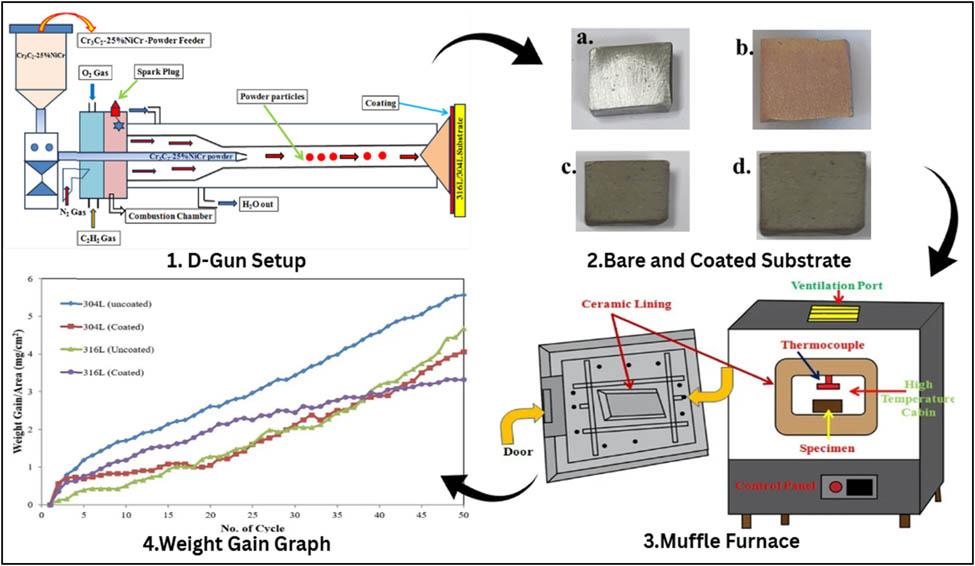
Figure 1
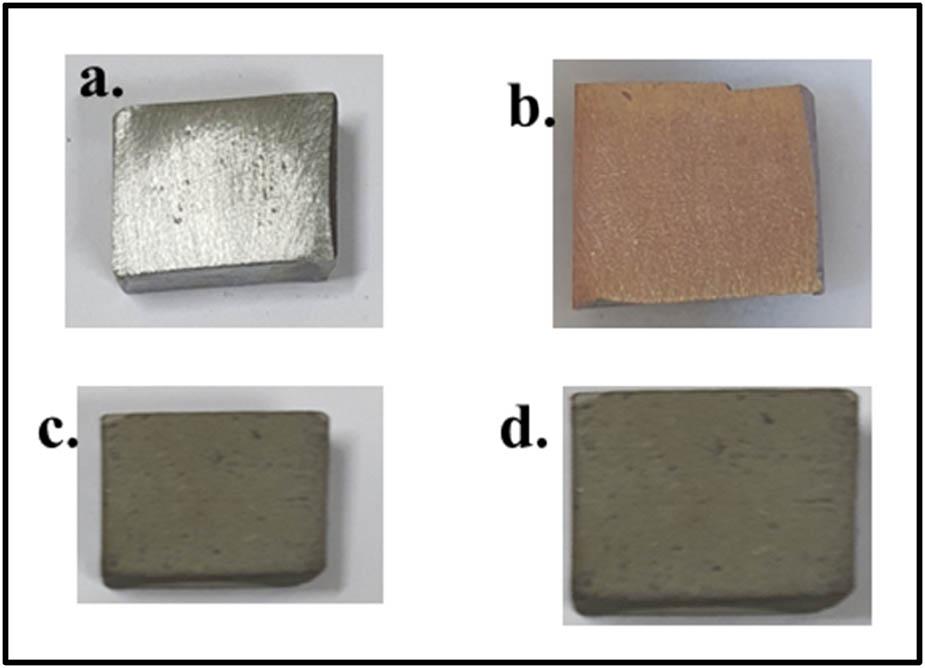
Figure 2
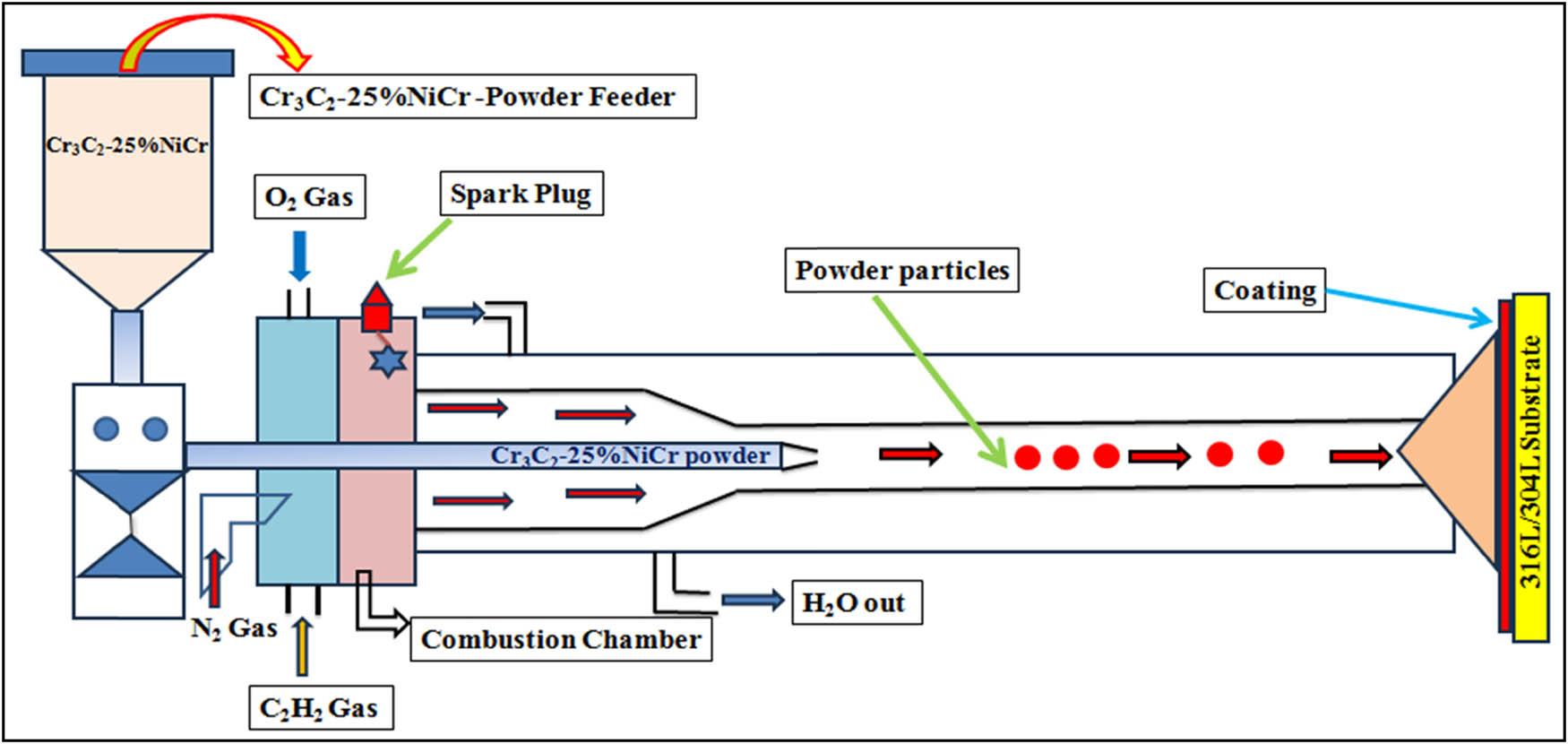
Figure 3
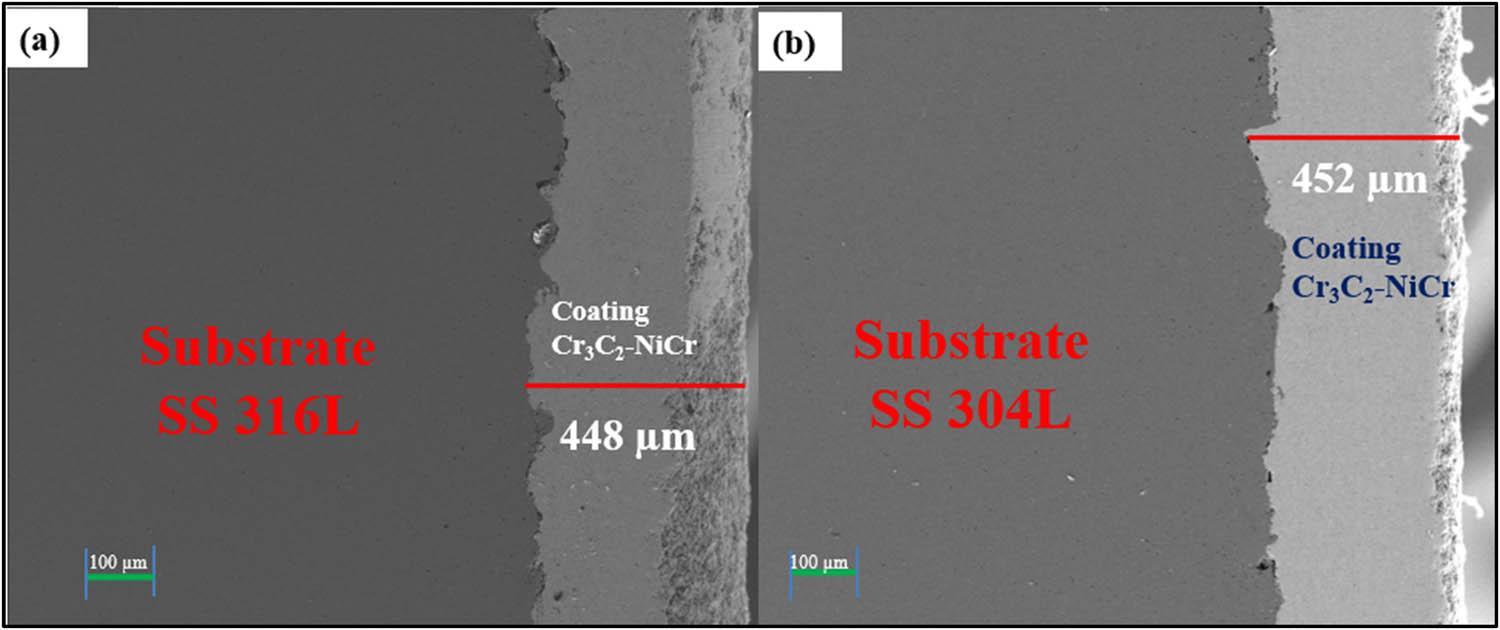
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8

Figure 9
Figure 10
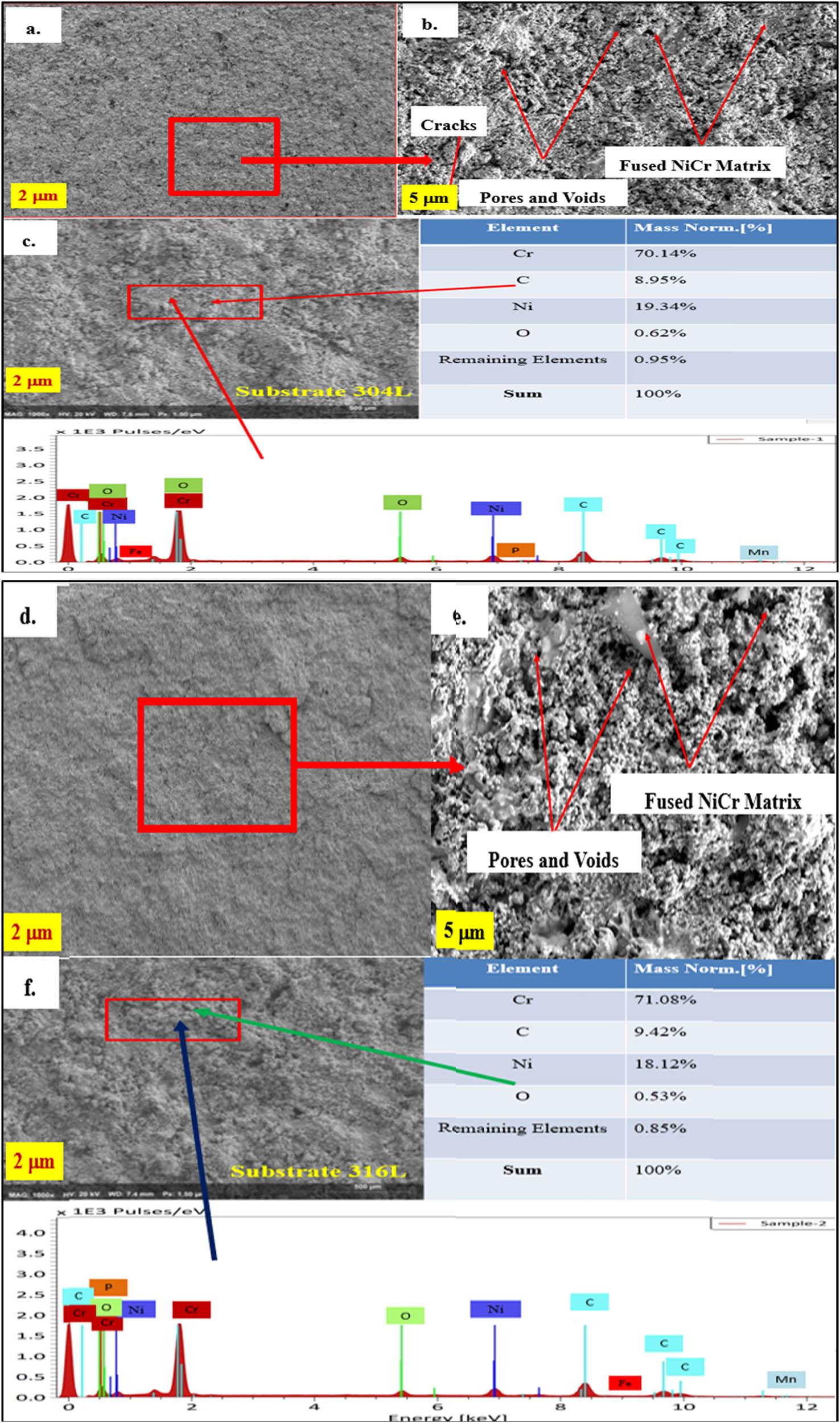
Figure 11
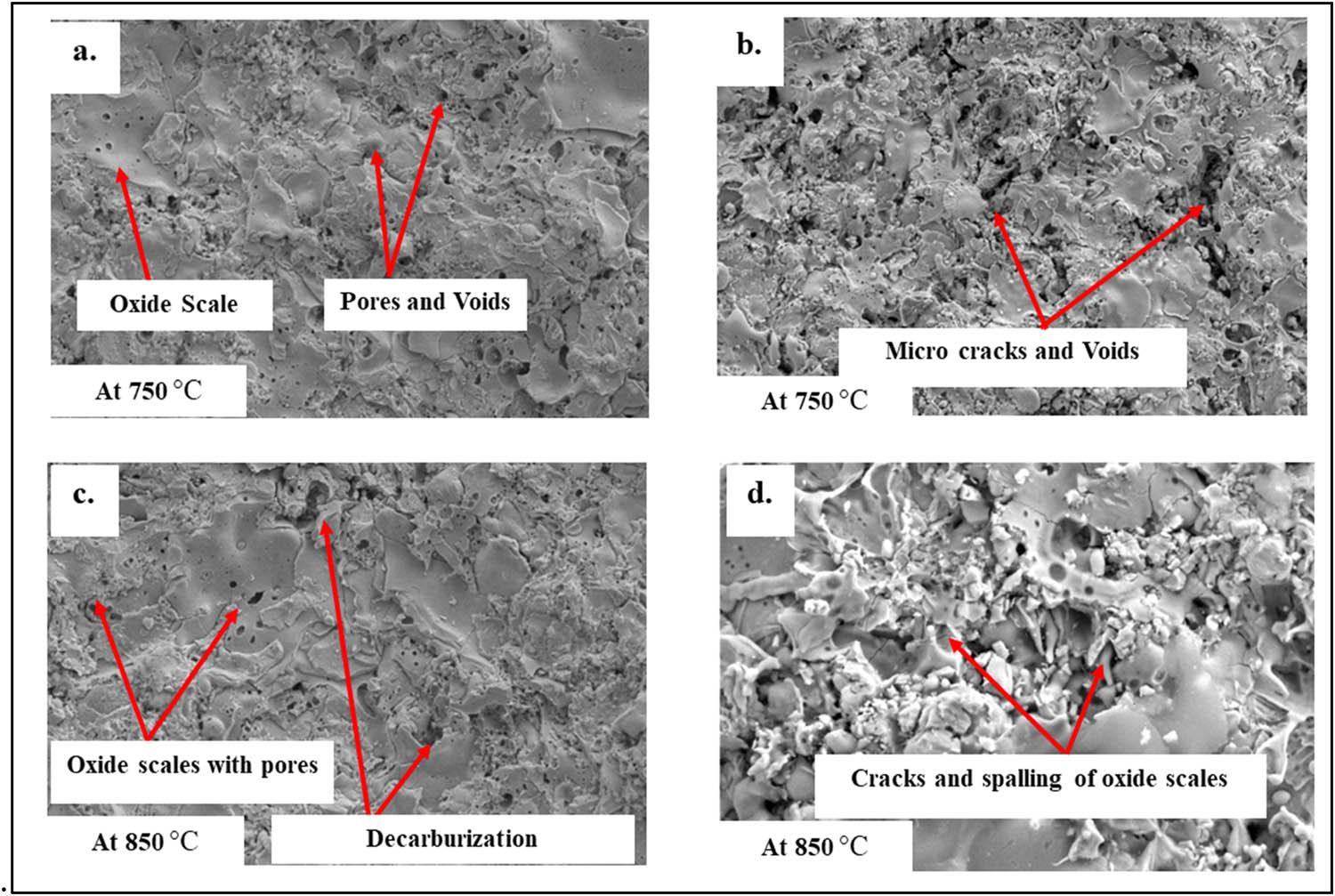
Figure 12
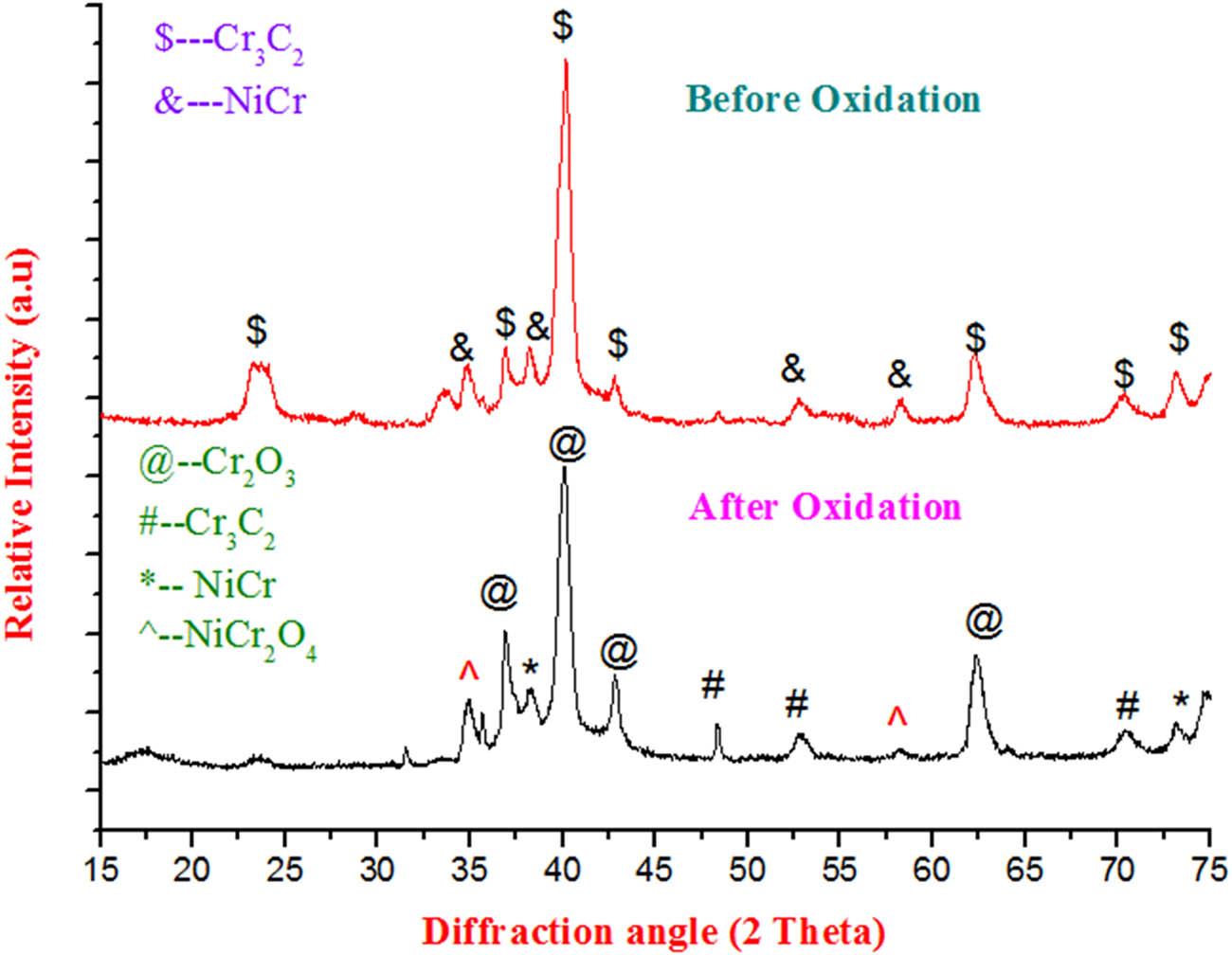
Powder coating process parameters_
| Parameters | Cr3C2–NiCr coating |
|---|---|
| Proportion | 75% Cr3C2/25% NiCr |
| Oxygen flow rate (O2) | 2720 SLPH |
| Acetylene (C2H2) flow rate | 2320 SLPH |
| Pressure (P) | 0.2 MPa |
| Nitrogen flow rate (N2) | 720 SLPH |
| Pressure (P) | 0.14 MPa |
| Power | 450 VA |
| Spray distance | 165 mm |
| Spray angle | 90o |
| Coating thickness (average) | 450 µm |
| Fire rate | 10 Hz (10 shots per second) |
304L and 316L SS chemical composition (%)_
| Substrate | C | Mn | P | S | Si | Cr | Ni | Mo | Fe% |
|---|---|---|---|---|---|---|---|---|---|
| SS 304L | 0.030 | 2.00 | 0.45 | 0.03 | 1.00 | 18.0–20.0 | 8.00–12.00 | — | Bal. |
| SS 316L | 0.030 | 2.00 | 0.45 | 0.03 | 1.00 | 16.0–18.0 | 10.00–14.00 | 2.00–3.00 | Bal. |