Fig. 1.
![Synthesis route of [2-(3,4-epoxycyclohexyl) ethyl] triphenylsilane](https://sciendo-parsed.s3.eu-central-1.amazonaws.com/666047fedd1c3d1f87135322/j_msp-2024-0020_fig_001.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Credential=AKIA6AP2G7AKOUXAVR44%2F20251204%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20251204T131709Z&X-Amz-Expires=3600&X-Amz-Signature=fd402fb00aa1067f5e693db48df9a22bcf7e95875544bed22ad74da2a4a40796&X-Amz-SignedHeaders=host&x-amz-checksum-mode=ENABLED&x-id=GetObject)
Fig. 2.
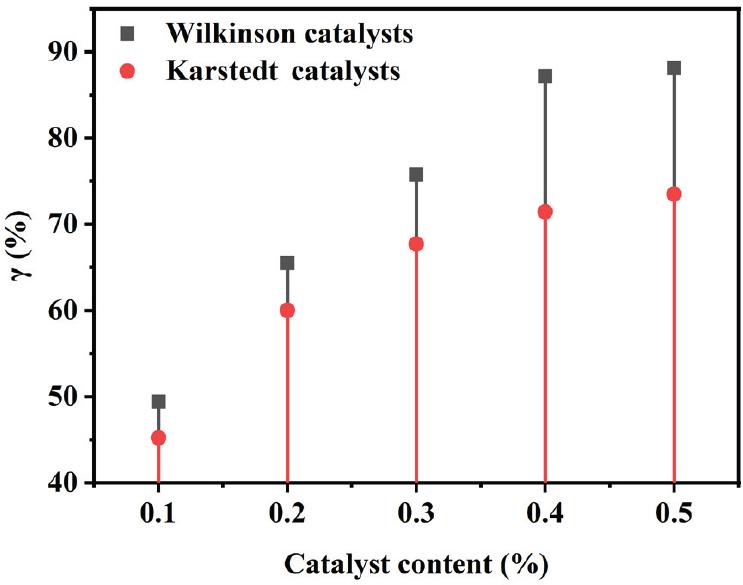
Fig. 3.
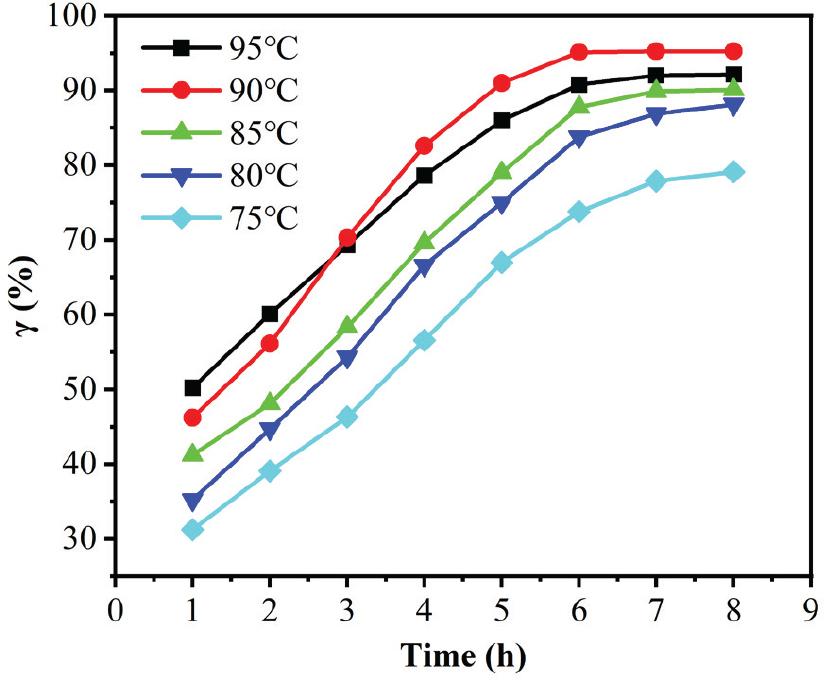
Fig. 4.
![FTIR spectra of triphenylsilane, 1,2-epoxy-4-vinylcyclohexane, [2-(3,4-epoxycyclohexyl) ethyl] triphenylsilane](https://sciendo-parsed.s3.eu-central-1.amazonaws.com/666047fedd1c3d1f87135322/j_msp-2024-0020_fig_004.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Credential=AKIA6AP2G7AKOUXAVR44%2F20251204%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20251204T131709Z&X-Amz-Expires=3600&X-Amz-Signature=3b050ebf86c6ac2d44aa87e37c14a22671b8eff4607927ca7f524b604ab1a58b&X-Amz-SignedHeaders=host&x-amz-checksum-mode=ENABLED&x-id=GetObject)
Fig. 5.
![1H -NMR hydrogen spectra of TPS, ECV, [2-(3,4-epoxycyclohexyl) ethyl] triphenylsilane](https://sciendo-parsed.s3.eu-central-1.amazonaws.com/666047fedd1c3d1f87135322/j_msp-2024-0020_fig_005.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Credential=AKIA6AP2G7AKOUXAVR44%2F20251204%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20251204T131709Z&X-Amz-Expires=3600&X-Amz-Signature=94567176582d9bcab45a99931fbe6022458c16a31c21b56447c0761408383c95&X-Amz-SignedHeaders=host&x-amz-checksum-mode=ENABLED&x-id=GetObject)
Fig. 6.
![1H-NMR pattern of [2-(3,4-epoxycyclohexyl)ethyl] triphenylsilane](https://sciendo-parsed.s3.eu-central-1.amazonaws.com/666047fedd1c3d1f87135322/j_msp-2024-0020_fig_006.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Credential=AKIA6AP2G7AKOUXAVR44%2F20251204%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20251204T131709Z&X-Amz-Expires=3600&X-Amz-Signature=e8db8b72a8cd9f346a56567d08e2911379b0d092e453d076a2b3801c4510b4b6&X-Amz-SignedHeaders=host&x-amz-checksum-mode=ENABLED&x-id=GetObject)
Fig. 7.
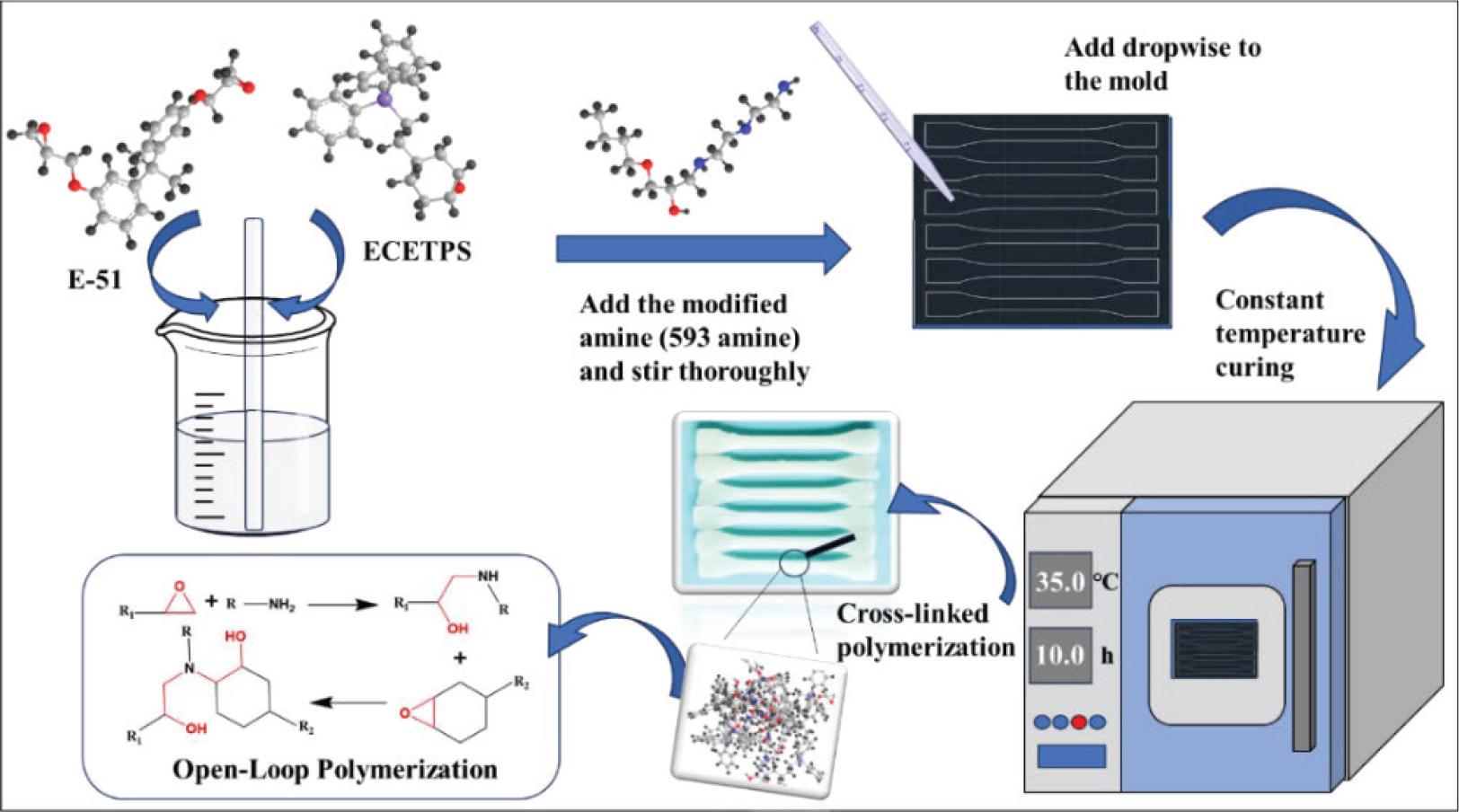
Fig. 8.
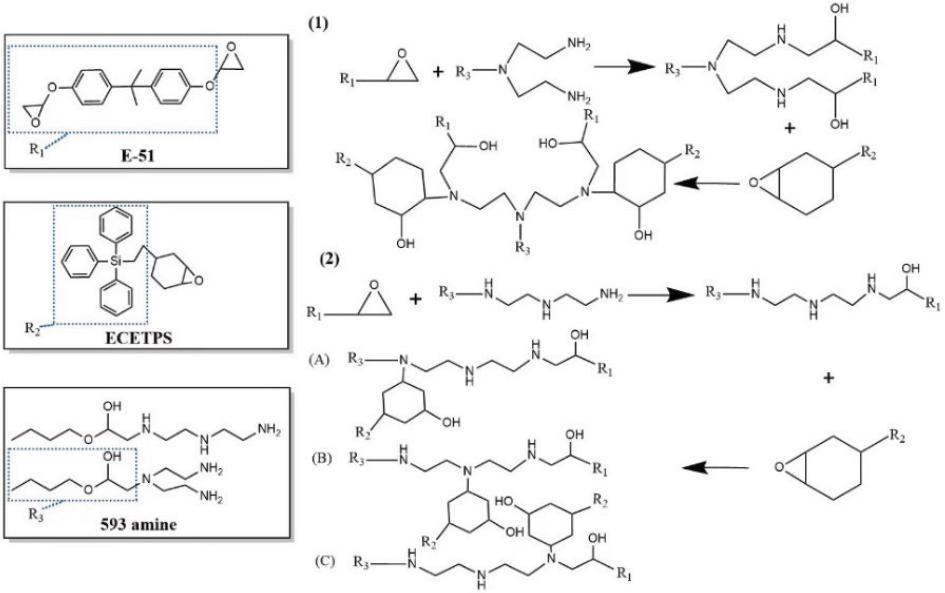
Fig. 9.
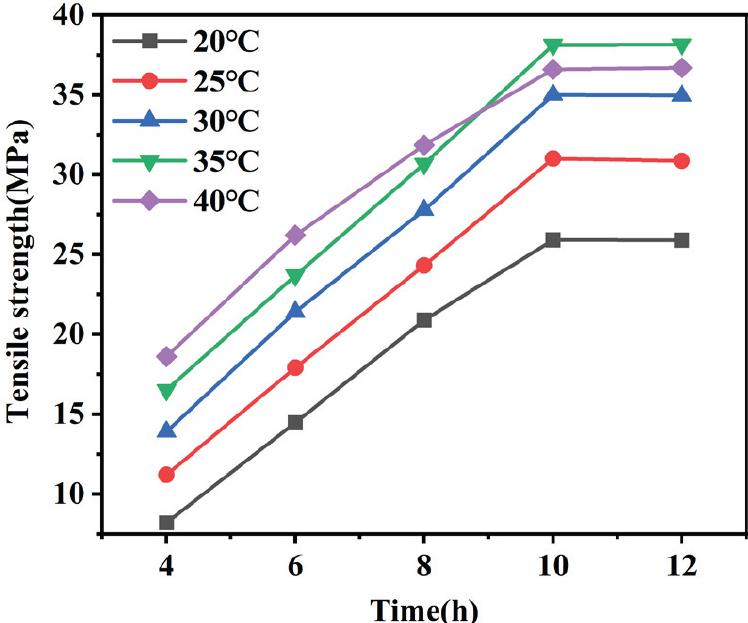
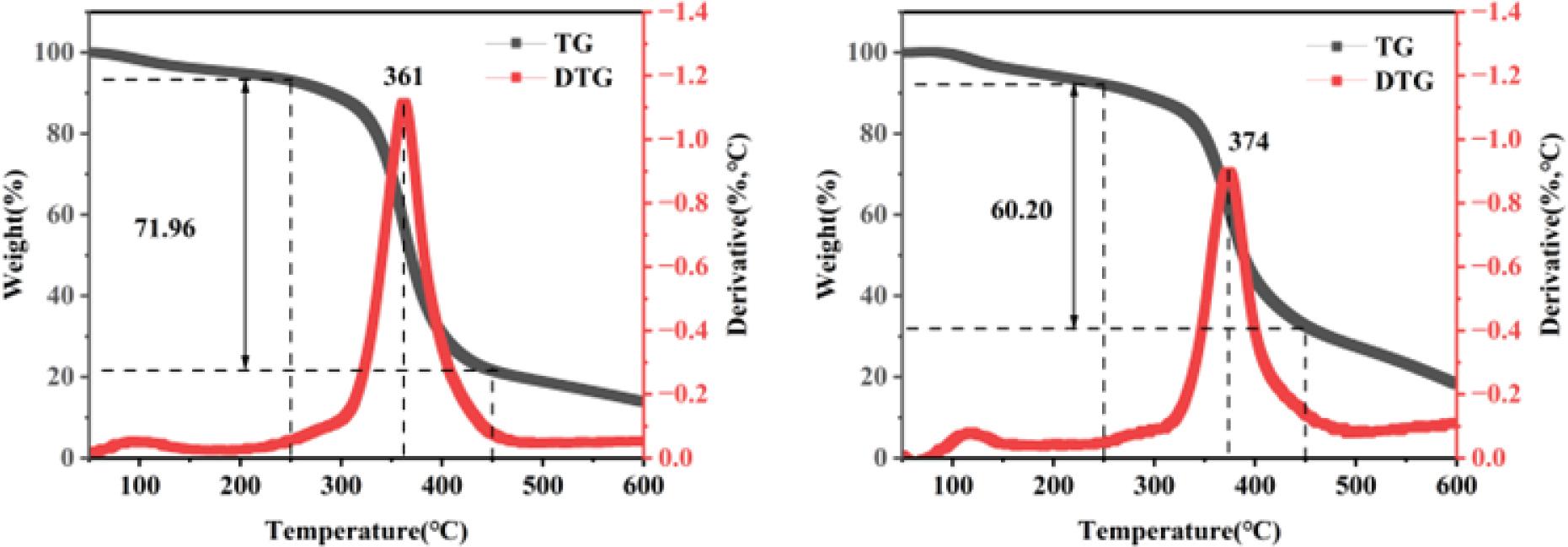
Fig. 10.
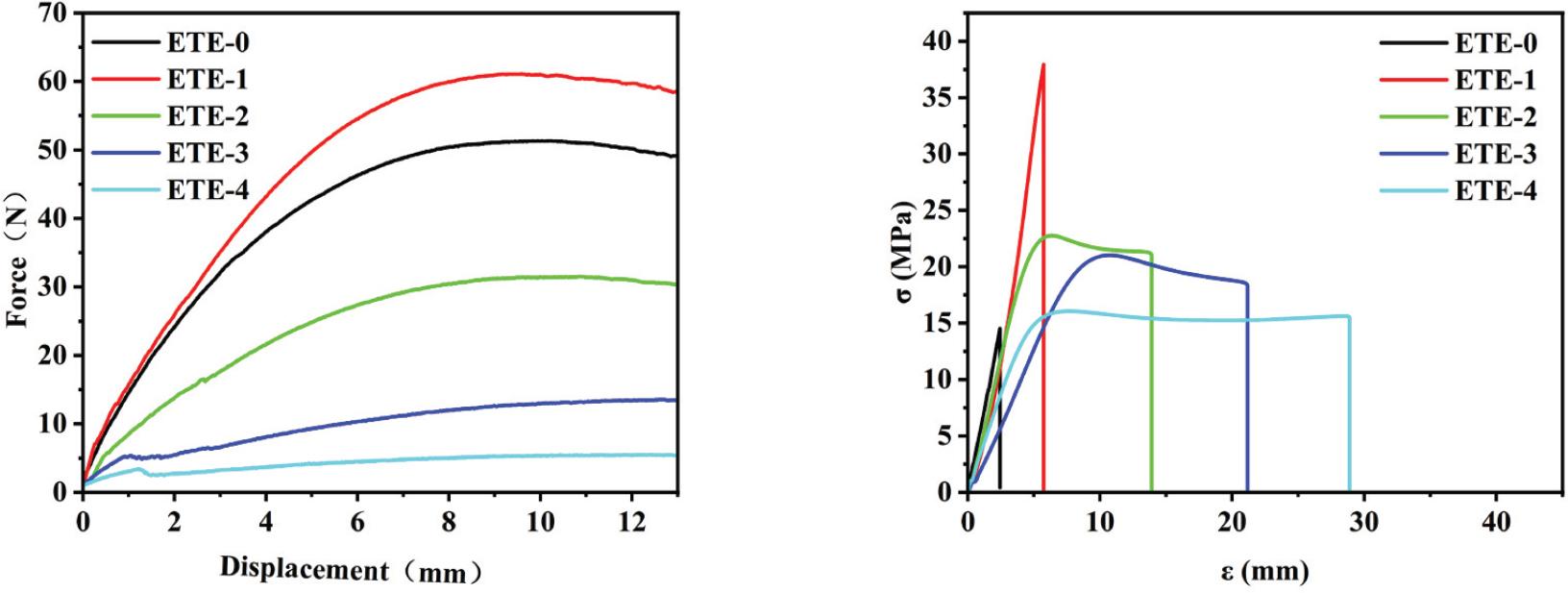
Fig. 11.
ECETPS yields based on different ratios
| Numbering | TPS:ECV | ECETPS |
|---|---|---|
| 1 | 1:1.10 | 81.58% |
| 2 | 1:1.15 | 83.70% |
| 3 | 1:1.20 | 92.24% |
| 4 | 1:1.25 | 92.34% |
| 5 | 1:1.30 | 92.61% |
Bending mechanical property data for cured samples
| Numbering | Elastic modulus (MPa) | Bending strength (MPa) | Elongation at break (%) |
|---|---|---|---|
| ETE-0 | 1136.65 | 25.38 | 8.31 |
| ETE-1 | 1847.91 | 39.10 | 9.73 |
| ETE-2 | 1342.19 | 20.24 | 8.93 |
| ETE-3 | 573.75 | 8.71 | 8.25 |
| ETE-4 | 239.12 | 3.06 | 7.74 |
Tensile mechanical property data for cured samples
| Numbering | Elastic modulus (MPa) | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|---|
| ETE-0 | 505.65 | 14.53 | 2.33 |
| ETE-1 | 581.22 | 37.95 | 4.00 |
| ETE-2 | 443.08 | 22.78 | 12.42 |
| ETE-3 | 384.06 | 20.60 | 19.36 |
| ETE-4 | 290.10 | 16.08 | 26.59 |
1H-NMR spectral analysis of ECETPS
| Location of H | Chemical shift/ (δ/ppm) | Integral ratio | Structural formula |
|---|---|---|---|
| 1 | 3.15 | 2.00 | 2H -O-CH- |
| 2 | 1.40~1.70 | 5.90 | 6H-CH-CH2-CH- |
| 3 | 1.72 | 1.17 | 1H -CH- |
| 4 | 1.17 | 1.98 | 2H C-CH2-CH2- |
| 5 | 2.24 | 2.37 | 2H Si-CH2-CH2- |
| 6 | 7.57 | 6.21 | 6H Si-C-CH- |
| 7 | 7.41 | 9.22 | 9H -CH-CH-CH- |
Amine curing ratios
| Numbering | ECETPS:E-51:593 amine |
|---|---|
| ETE-0 | 0.0:8.0:2.0 |
| ETE-1 | 0.8:7.2:2.0 |
| ETE-2 | 1.6:6.4:2.0 |
| ETE-3 | 2.4:5.6:2.0 |
| ETE-4 | 3.2:4.8:2.0 |