Figure 1.

Figure 2.
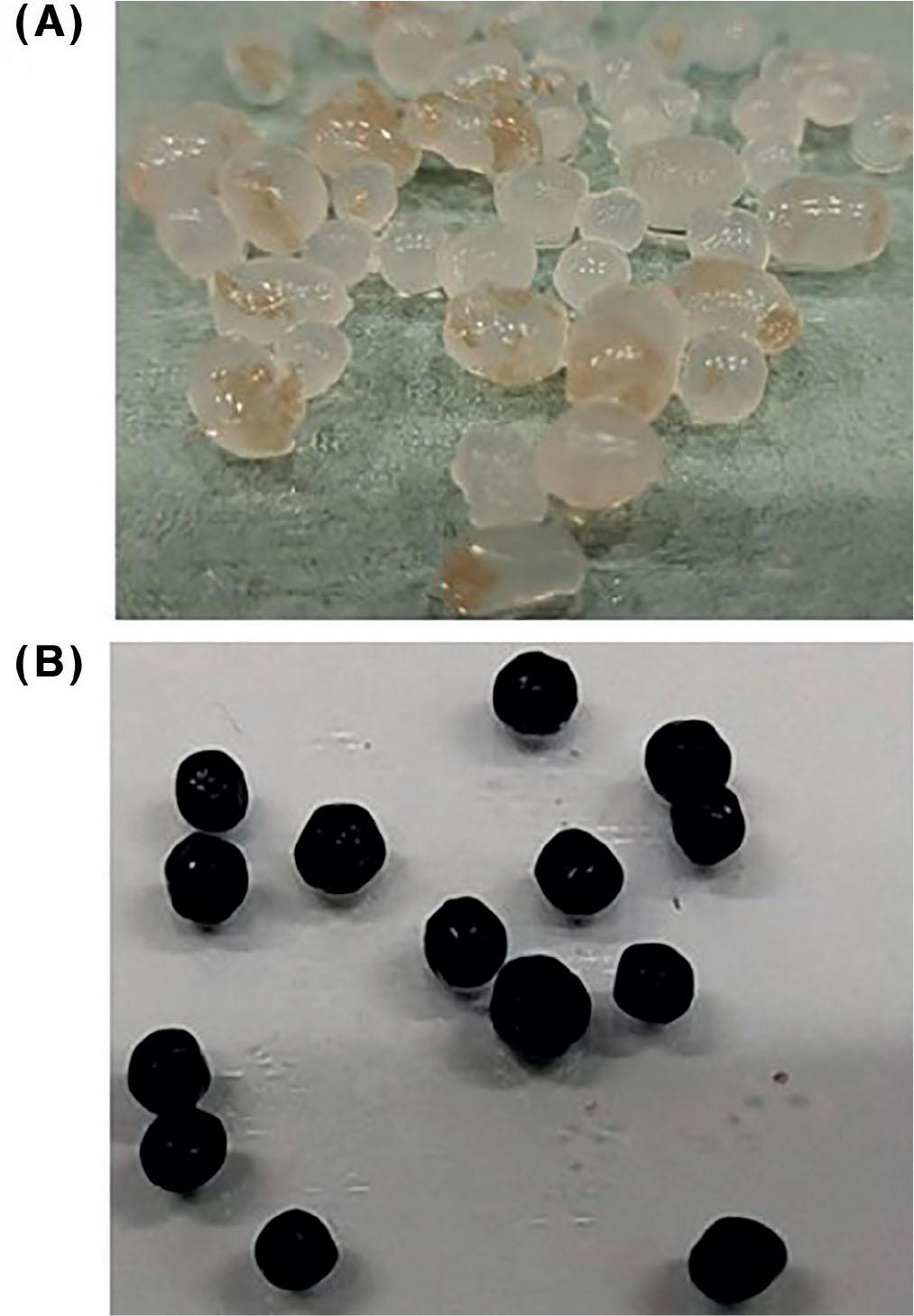
Figure 3.

Figure 4.
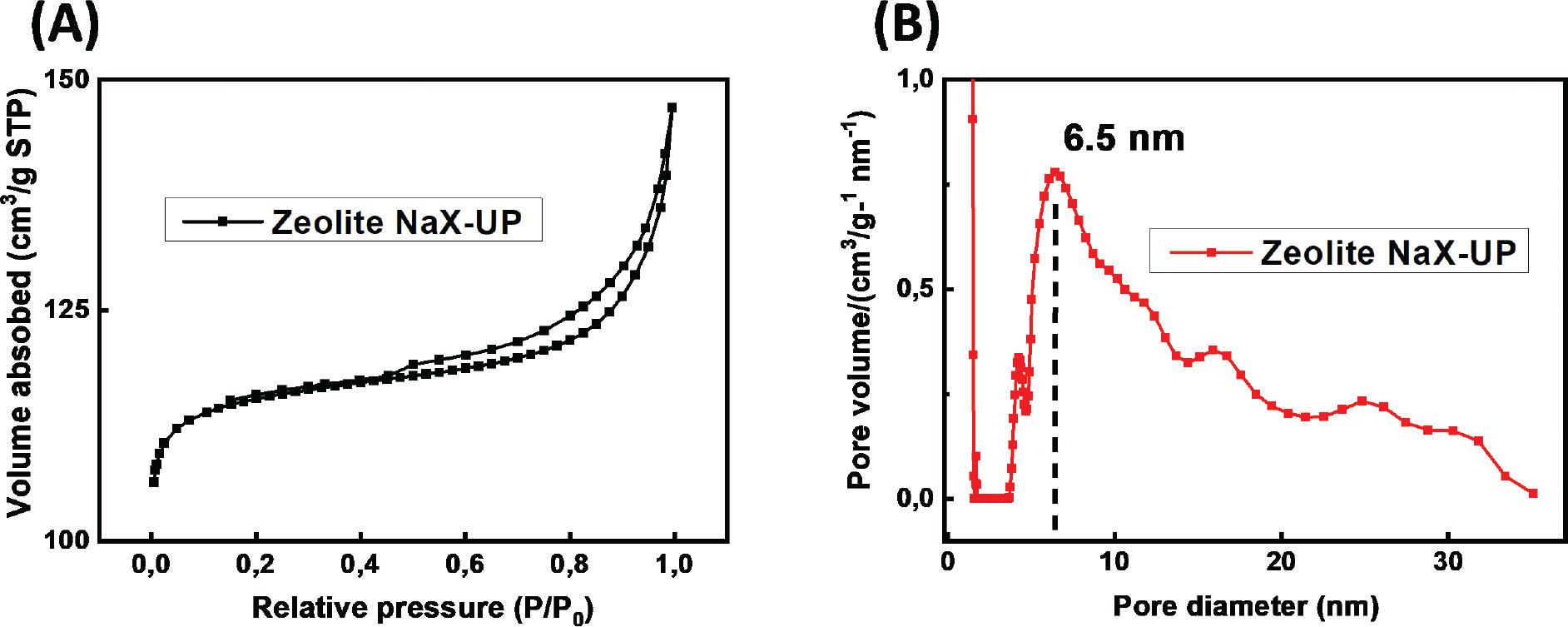
Figure 5.
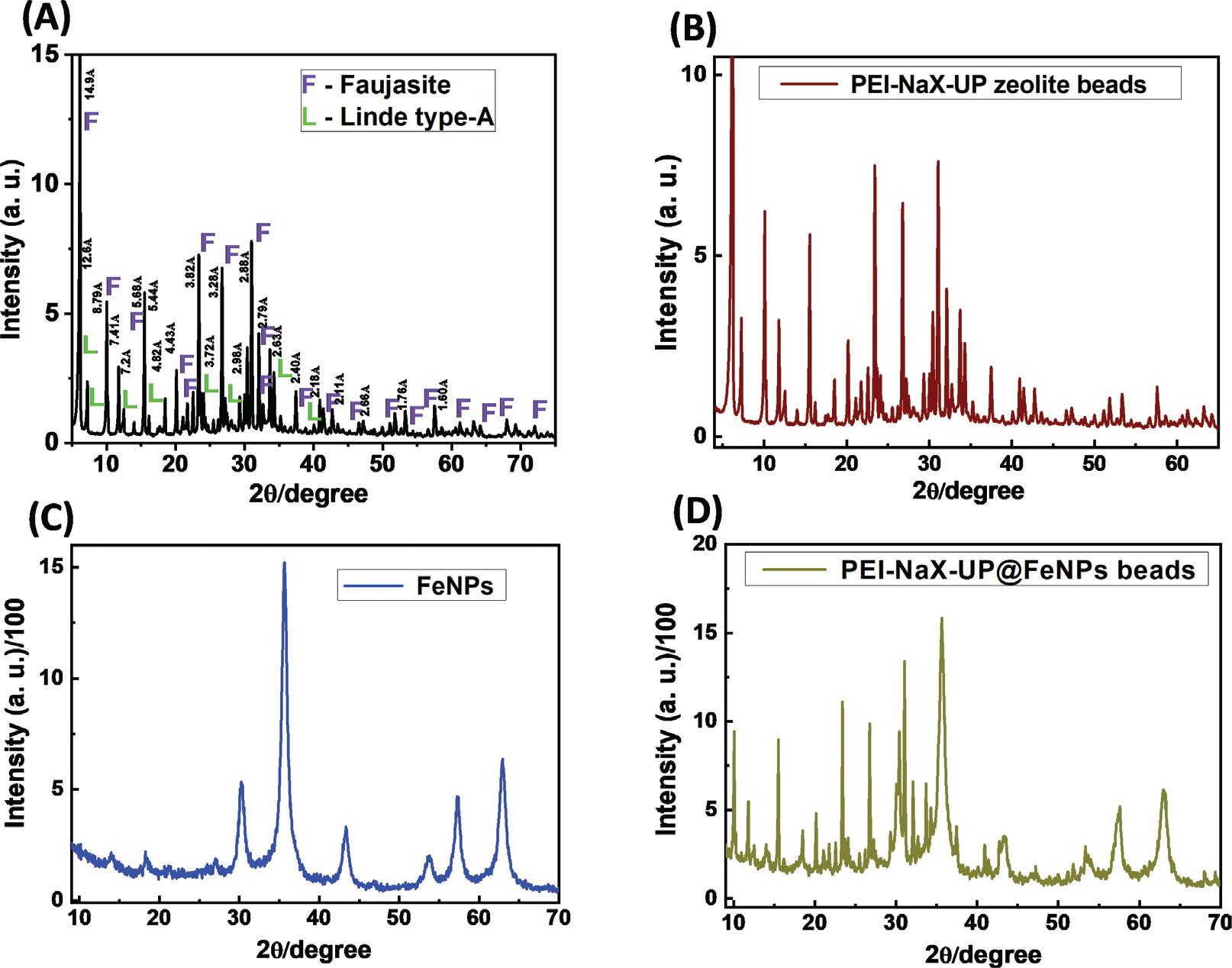
Figure 6.
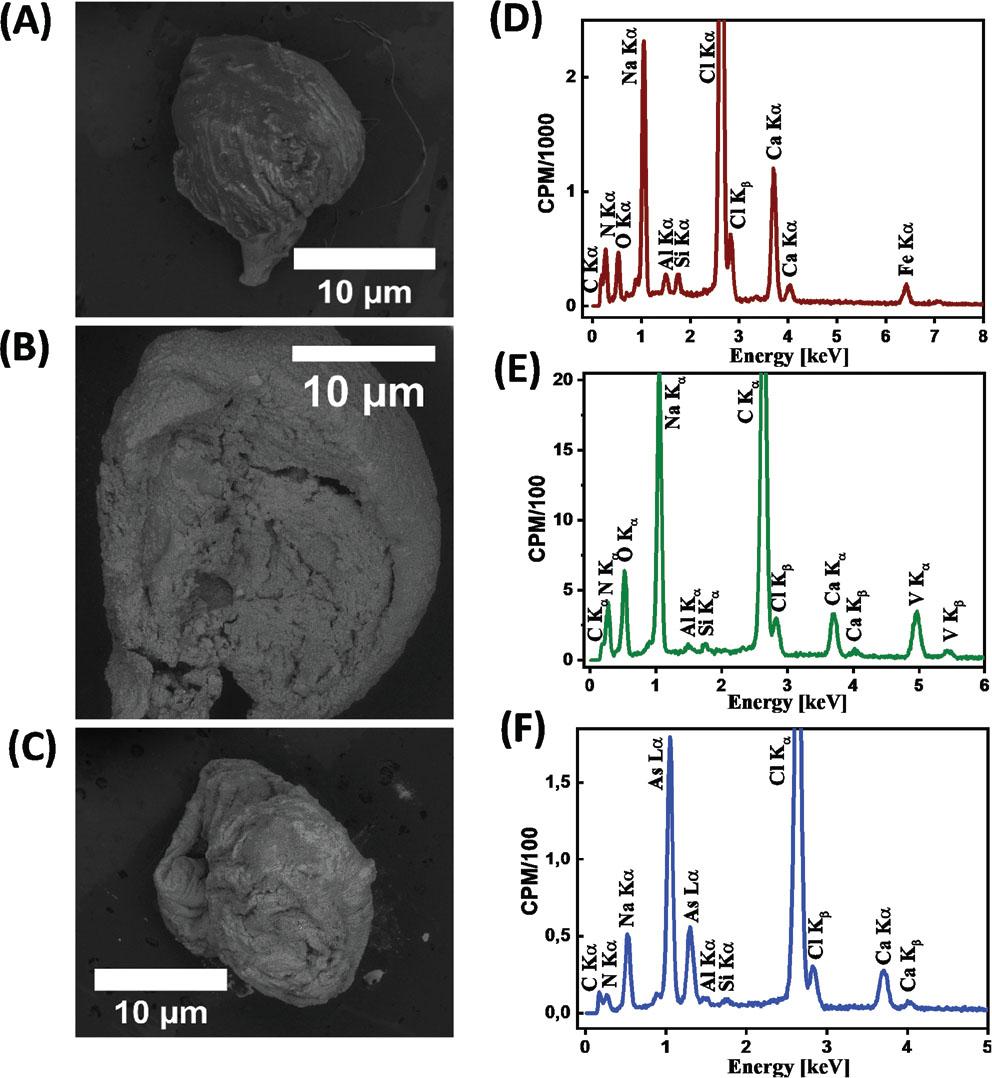
Figure 7.

Figure 8.

Elemental compositions determined by XRF method_
| S. No. | Composition | XRF [% weight] |
|---|---|---|
| 1 | SiO2 | 32.05 |
| 2 | TiO2 | 0.04 |
| 3 | Al2O3 | 27.07 |
| 4 | Fe2O3 | 0.40 |
| 5 | MnO | 0.08 |
| 6 | MgO | 1.16 |
| 7 | CaO | 5.32 |
| 8 | K2O | 0.52 |
| 9 | Na2O | 8.81 |
| 10 | P2O5 | 0.07 |
| 11 | SO3 | 0.10 |
| 12 | LOI | 24.19 |
| Σ | 99.81 |
Arsenic (V) adsorption capacity of various adsorbents reported in the literature_
| Adsorption material | pH | qm (mg g-1) | Reference |
|---|---|---|---|
| Alginate@Iron-zirconium@Zeolite W | 2.0 | 42.3 | Abdellaoui et al. 2021 |
| Chitosan-coated Na–X zeolite | 2.0 | 63.2 | Han et al. 2019 |
| Zeolite@nanoscale zero-valent iron | 3.0 | 38.2 | Suazo-Hernandez et al. 2019 |
| Na-X zeolite derived from cola fly ash | 2.14 | 27.7 | Yang et al. 2019 |
| Nanozero valent iron (NZVI) loaded on zeolite 5A | 4-12 | 72.0 | Yang et al. 2022. |
| β-cyclodextrin modified graphene oxide @ Fe3O4 NPs | 4-7.2 | 99.5 | Santhana Krishna Kumar et al. 2017 |
| Iron oxide (hydr) modified zeolite | 3.5 | 1.69 | Nekhunguni et al. 2017 |
| Ferric hydroxide microcapsule-loaded alginate beads | - | 3.80 | Sarkar et al. 2010 |
| Calcium alginate beads@methionine magnetic NPs | 7-7.5 | 6.6 | Lilhare et al. 2021 |
| Aluminum(III) exchange synthetic zeolite | 3-10 | 10.5 | Xu et al. 2002 |
| Fe-treated synthetic zeolite | - | 22.5 | Dousova et al. 2006 |
| Cerium(III) exchange synthetic zeolite | - | 23.4 | Haron et al. 2008 |
| Synthetic zeolites | 3.2 | 35.8 | Chutia et al. 2009 |
| PEI-modified zeolite beads | 5-7.5 | 35.4 | This study |
| PEI-modified Fe3O4 beads | 5-7.5 | 88.4 | This study |
| Fe3O4 decorated PEI-modified zeolite beads | 5-7.5 | 163.9 | This study |
Adsorption kinetics for uptake of Arsenic(V) and Vanadium(V)
| Sl. No | Adsorbent | Order of kinetics | Characteristic values | Uptake of As(V) | Uptake of Vanadium(V) |
|---|---|---|---|---|---|
| 1 | PEI-modified zeolite beads | Pseudo first order | qe (mg g-1) | 1.36 | 1.28 |
| k1 | 0.007 | 0.018 | |||
| Adj. R2 | 0.92 | 0.94 | |||
| 2 | PEI-modified zeolite beads | Pseudo second order | qe (mg g-1) | 28.4 | 23.2 |
| k2 | 0.0005 | 0.0008 | |||
| Adj R2 | 0.98 | 0.98 | |||
| 3 | PEI-modified Fe3O4 beads | Pseudo second order | qe (mg g-1) | 67.1 | 46.5 |
| k2 | 0.0002 | 0.0004 | |||
| Adj R2 | 0.98 | 0.98 | |||
| 4 | PEI-modified Fe3O4 beads | Pseudo first order | qe (mg g-1) | 1.66 | 1.58 |
| k1 | 0.016 | 0.019 | |||
| Adj. R2 | 0.96 | 0.94 | |||
| 5 | Fe3O4 decorated PEI-modified | Pseudo first order | qe (mg g-1) | 1.86 | 1.72 |
| zeolite beads | k1 | 0.014 | 0.02 | ||
| Adj. R2 | 0.94 | 0.94 | |||
| 6 | Fe3O4 decorated PEI-modified | Pseudo second order | qe (mg g-1) | 99.0 | 77.5 |
| zeolite beads | k2 | 0.0001 | 0.0002 | ||
| Adj R2 | 0.98 | 0.98 |
Adsorption isotherm parameters for uptake of Arsenic(V) and Vanadium(V)_
| Sl. No. | Adsorbent | Isotherm | Characteristic values | Uptake of As(V) | Uptake of V(V) |
|---|---|---|---|---|---|
| 1 | PEI-modified zeolite beads | Langmuir | qm (mg g-1) | 35.4 | 38.9 |
| b (L mg-1) | 0.014 | 0.014 | |||
| RL | 0.69 | 0.69 | |||
| Adj. R2 | 0.96 | 0.96 | |||
| 2 | PEI-modified zeolite beads | Freundlich | KF (mg 1-1/n g-1 L 1/n) | 0.40 | 0.44 |
| n | 1.08 | 1.08 | |||
| Adj R2 | 0.98 | 0.98 | |||
| 3 | PEI-modified Fe3O4 beads | Freundlich | KF (mg1-1/ng-1L1/n) | 1.008 | 0.68 |
| n | 1.08 | 1.02 | |||
| Adj R2 | 0.98 | 0.98 | |||
| 4 | PEI-modified Fe3O4 beads | Langmuir | qm (mg g-1) | 88.4 | 53.1 |
| b (L mg-1) | 0.01 | 0.02 | |||
| RL | 0.57 | 0.30 | |||
| Adj. R2 | 0.96 | 0.96 | |||
| 5 | Fe3O4 decorated PEI-modified zeolite beads | Langmuir | qm (mg g-1) | 163.9 | 151.5 |
| b (L mg-1) | 0.02 | 0.02 | |||
| RL | 0.25 | 0.25 | |||
| Adj. R2 | 0.96 | 0.96 | |||
| 6 | Fe3O4 decorated PEI-modified zeolite beads | Freundlich | KF (mg1-1/ng-1L1/n) | 2.09 | 1.93 |
| n | 1.08 | 1.08 | |||
| Adj R2 | 0.98 | 0.98 |
Arsenic (V) equilibrium kinetics sorption, adsorption capacity of various adsorbents reported in the literature_
| Adsorption material | Equilibrium kinetics (Time in min) | qm (mg g-1) | Reference |
|---|---|---|---|
| Chitosan-coated Na–X zeolite | 720 min | 63.2 | Han et al. 2019 |
| Na-X zeolite derived from cola fly ash | 120 min | 27.7 | Yang et al. 2019 |
| Iron oxide (hydr) modified zeolite | 90 min | 1.69 | Nekhunguni et al. 2017 |
| Calcium alginate beads@Methionine magnetic NPs | 110 min | 6.6 | Lilhare et al. 2021 |
| Aluminum(III) exchange synthetic zeolite | 960 min | 10.5 | Xu et al. 2002 |
| Synthetic zeolites | 100 min | 35.8 | Chutia et al. 2009 |
| PEI-modified zeolite beads | 100 min | 35.4 | This study |
| PEI-modified Fe3O4 beads | 100 min | 88.4 | This study |
| Fe3O4 decorated PEI-modified zeolite beads | 100 min | 163.9 | This study |
Vanadium equilibrium kinetics sorption, adsorption capacity of various adsorbents reported in the literature_
| Adsorption material | Equilibrium kinetics (Time in hours) | qm (mg g-1) | Ref |
|---|---|---|---|
| Magnetic zeolite–polypyrrole composite | 4 hours | 74.9 | Mthombeni et al. 2016 |
| Goethite | 12 hours | 8.2 | Zhu et al. 2020 |
| Clinoptilolite modified polypyrrole and iron NPs | 14 hours | 12.0 | Mthombeni et al. 2018 |
| Synthesised zero-valent iron modified kaloin | 2 hours | 15.0 | Bello et al. 2019 |
| PGTFS-NH3+Cl- | 4 hours | 45.9 | Anirudhan et al. 2010 |
| PEI-modified zeolite beads | 1.5 hours | 38.9 | This study |
| PEI-modified Fe3O4 beads | 1.5 hours | 53.1 | This study |
| Fe3O4 decorated PEI-modified zeolite beads | 1.5 hours | 151.5 | This study |
Vanadium adsorption capacity of various adsorbents reported in the literature_
| Adsorption material | pH | qm (mg g-1) | Reference |
|---|---|---|---|
| LDH supported nanoscale zero valent iron | 3.0 | 93.7 | Kong et al. 2020 |
| Magnetic zeolite–polypyrrole composite | 4-5 | 74.9 | Mthombeni et al. 2016 |
| Syrian natural zeolite | 6.0 | 40.0 | Salman et al. 2017 |
| Goethite | 4-8 | 8.2 | Zhu et al. 2020 |
| Clinoptilolite modified polypyrrole and iron oxide NPs | 4.5 | 12.0 | Mthombeni et al. 2018 |
| Synthesised zero-valent iron modified kaloin | 5.0 | 15.0 | Bello et al. 2019 |
| Octylamine functionalized magnetite NPs | 3.2 | 25.7 | Parijaee et al. 2014 |
| Zeolite A | 4.5 | 30.0 | Mahmood et al. 2019 |
| PGTFS-NH3+Cl- | 6.0 | 45.9 | Anirudhan et al. 2010 |
| Biochar stabilized nano zero-valent iron | 2-10 | 48.5 | Fan et al. 2020 |
| Metal (hydr)oxide adsorbents | 3-4 | 111.1 | Naeem et al. 2007 |
| PEI-modified zeolite beads | 5-7.5 | 38.9 | This study |
| PEI-modified Fe3O4 beads | 5-7.5 | 53.1 | This study |
| Fe3O4 decorated PEI-modified zeolite beads | 5-7.5 | 151.5 | This study |
Adsorption of thermodynamics for uptake of As(V) and V(V)_
| S. No. | Adsorbent | Uptake of metals | ΔS0 J mol-1 | ΔH0 KJ mol-1 | ΔG0 KJ mol-1 | |||
|---|---|---|---|---|---|---|---|---|
| 298K | 308K | 318K | 328K | |||||
| 1 | PEI-Zeolite NSs | As(V) | 145.7 | 42.5 | −2.1245 | −3.9625 | −5.2465 | −6.8752 |
| beads | V(V) | 124.8 | 39.1 | −1.4523 | −2.2754 | −3.1465 | −4.1254 | |
| 2 | PEI-Fe3O4 NPs | As(V) | −121.3 | −37.2 | −6.8875 | −5.2354 | 4.26548 | −2.1256 |
| beads | V(V) | −91.3 | −26.7 | −4.2810 | −3.8965 | −2.23689 | −1.4689 | |
| 3 | PEI-Zeolite NSs@ | As(V) | −163.1 | −54.8 | −8.3567 | −7.3658 | −5.2136 | −2.8969 |
| Fe3O4 NPs beads | V(V) | −126.4 | −50.3 | −5.1209 | −3.6875 | −1.4232 | −2.0256 | |
Surface area, pore volume and pore diameter of NaX-UP zeolites_
| S. No. | SBET (m2 g-1) | Vtot0.99 (cm3 g-1) | VmikDR (cm3 g-1) | VmezBJH (cm3 g-1) |
|---|---|---|---|---|
| 1 | 472.0 | 0.222 | 0.176 | 0.13 |