Aortic stenosis (AS) is associated with significant morbidity and mortality. It affects about 4% of people over the age of 65, and its prevalence increases with age. Without appropriate treatment, the estimated survival time after symptom onset is 1 to 2 years.1 Transcatheter aortic valve implantation (TAVI) has become the preferred treatment for patients at high surgical risk in recent years, owing to its increased patient comfort and shorter recovery time compared with conventional surgery.2 The transfemoral approach is the recommended vascular access route, and the development of smaller-profile devices now allows approximately 90% of patients to undergo transfemoral TAVI, thereby reducing access-related complications.3
Nevertheless, several vascular complications may occur following TAVI, including hematoma, arterial dissection, arterial rupture, pseudoaneurysm, distal embolization, or major bleeding.4,5,6,7,8 To improve outcomes, Hayashida et al.9 proposed the sheath-to-femoral artery ratio (SFAR) as an index for predicting vascular complications, demonstrating that a higher SFAR was associated with increased rates of major complications and 30-day mortality.9 Leclercq et al.10 further reported that the surgical approach to transfemoral TAVI was associated with a lower rate of vascular complications.
The aim of paper is to present the surgical management and outcome of an elderly high-risk patient who developed an infected femoral pseudoaneurysm after TAVI, with a focus on the feasibility and effectiveness of neofemoral artery reconstruction using an autologous bifurcated great saphenous vein graft.
We present the case of an 88-year-old patient with a history of hypertension, severe aortic stenosis, valvular cardiomyopathy in the dilated stage, NYHA Class III congestive heart failure, chronic kidney disease, and generalized atheromatosis, who was hospitalized in the cardiology department for a TAVI procedure. On the first postoperative day, the patient reported severe pain in the right lower extremity with abrupt onset. Clinical examination revealed marbled, cold skin extending to the right thigh, with markedly reduced sensitivity and motility. Pulses of the femoral, popliteal, and below-the-knee arteries were absent in the right lower limb. An urgent computed tomography angiography (CTA) of the abdominal aorta and lower limbs revealed a patent right common iliac artery (CIA) and thrombotic occlusion of the external iliac artery (EIA), common femoral artery (CFA), and both superficial (SFA) and deep (PFA) branches of the right femoral artery (Figure 1).

Preoperative CTA with 3D reconstruction showing thrombosis of the right EIA, CFA, PFA, and SFA: suprainguinal (A) and infrainguinal (B) views.
The patient was admitted to the Vascular Surgery Clinic for surgical treatment. The procedure began with a right inguinal incision and exposure of the CFA, SFA, and PFA, which were suspended on loops. After systemic heparinization, the femoral arteries were clamped and a longitudinal arteriotomy was performed. Intraluminally, fresh thrombotic material was observed with complete absence of blood flow. A transfemoral thromboembolectomy using a Fogarty catheter was performed along the iliofemoral axis, supplemented by a local endarterectomy due to severe atherosclerotic lesions at the CFA bifurcation.
Finally, patch angioplasty of the CFA with extension to the SFA was carried out using a Dacron patch. Postoperatively, symptoms resolved, with restoration of the femoral pulse and Doppler signals at the popliteal and right pedal arteries, indicating preserved sensitivity and motility. Unfortunately, a reintervention was required 24 hours later to evacuate a right inguinal hematoma. The patient was transferred to the Cardiology Clinic on the second postoperative day for further medical management and monitoring.
After a 3-week interval from the previous procedure, the patient presented to the emergency department with dehiscence of the right groin wound, purulent discharge, and local pain. Surgical wound cleansing with mechanical debridement and contact drainage was performed. Bacteriological analysis revealed abundant growth of methicillin-sensitive Staphylococcus aureus (MSSA), which was treated according to the antibiogram during hospitalization, with continued therapy at home.
The patient did not attend the scheduled follow-up, and 3 months later returned to the emergency department with severe groin pain and active bleeding from the wound. CTA revealed a large infected pseudoaneurysm of the CFA, associated with a purulent collection, measuring approximately 4 × 2.5 cm (Figure 2).

Preoperative CTA demonstrating the pseudoaneurysm with active bleeding (yellow arrow) and the peri-pseudoaneurysmal infection area (asterisk).
The patient was admitted to the Vascular Surgery Clinic for surgical treatment. An elliptical incision was made in the right groin, followed by careful dissection of the CFA below the inguinal ligament and of the distal SFA, which were secured with vessel loops. After clamping the CFA and SFA, the pseudoaneurysm was opened longitudinally, and the PFA ostium was identified. Intraluminal clamping was then performed using a Fogarty catheter (Figure 3).

Intraoperative view after removal of the infected patch and preparation of the three femoral arteries for reconstruction.
After removal of the infected patch, the poor quality of the remaining arterial wall necessitated reconstruction of the CFA bifurcation. A neo-femoral bifurcation Y-shaped graft was fashioned from two segments of the great saphenous vein (GSV) (Figure 4A). The proximal anastomosis was performed with 5-0 Prolene, followed by partial declamping to verify graft patency and appearance (Figure 4B). Subsequently, anastomoses were completed at the PFA and SFA with selective declamping, patency verification, and local hemostasis (Figure 4C).
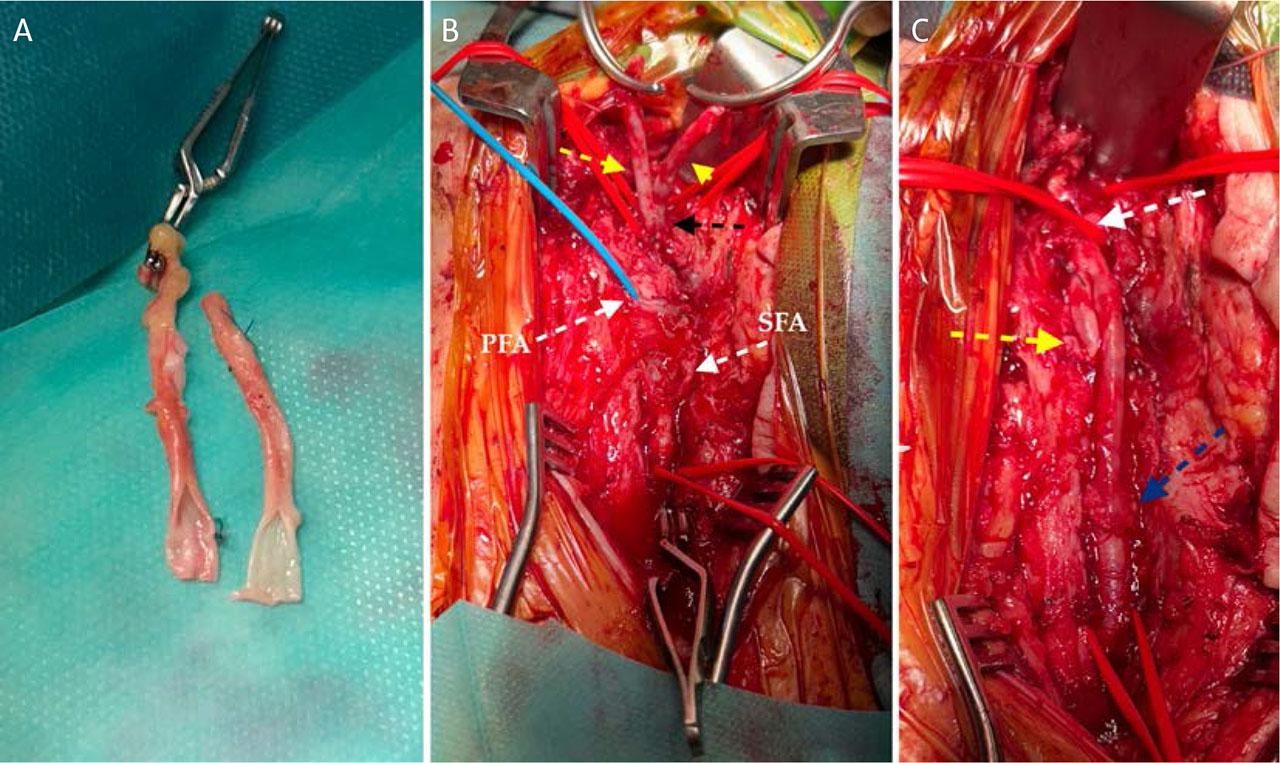
Intraoperative stages of neo-femoral artery reconstruction: creation of a Y-shaped graft from two GSV segments (A); proximal anastomosis with the CFA (B); and final appearance of the reconstruction (C).
To enhance the healing process, the defect was covered with a proximal sartorius muscle flap (Figure 5). Bacteriological examination of the excised Dacron patch identified infection with Staphylococcus epidermidis, which was treated according to the antibiogram.
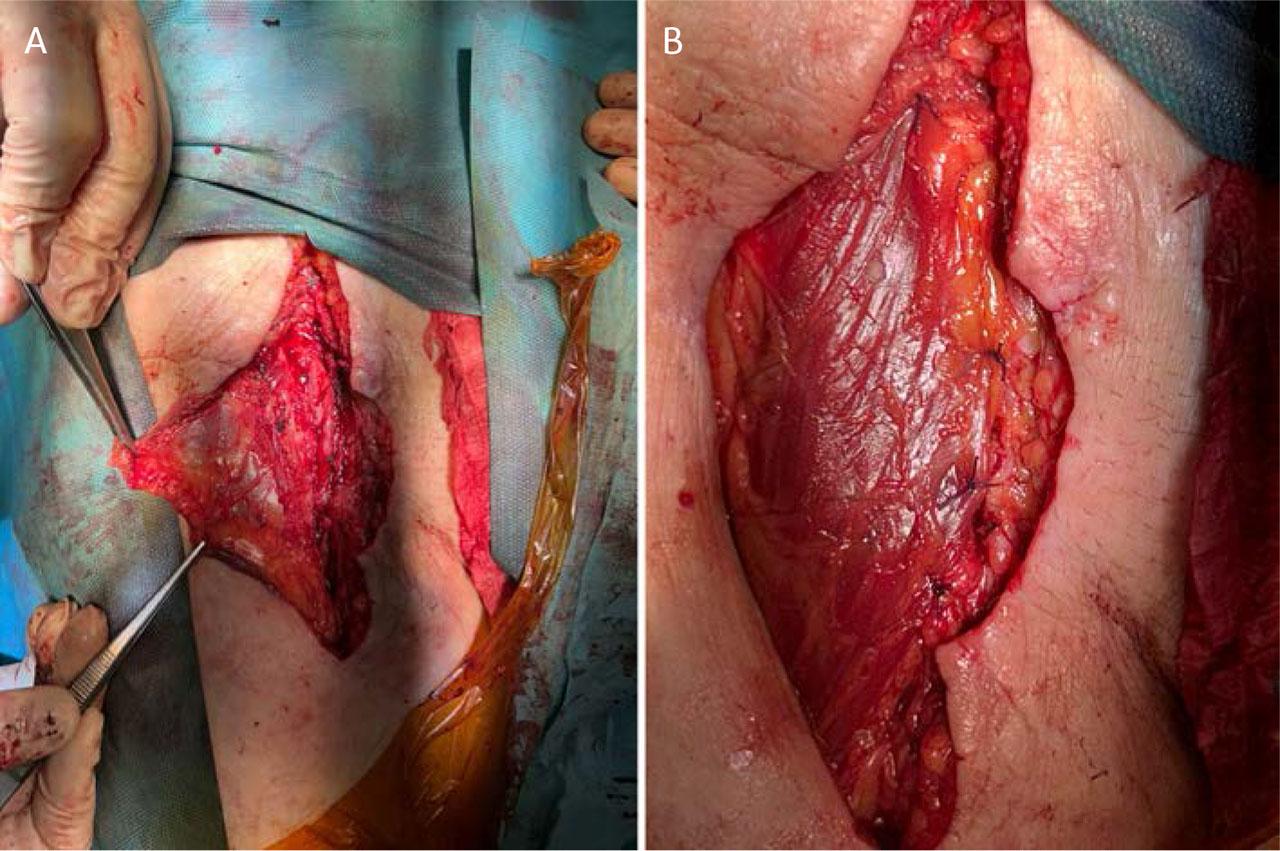
Coverage of the neo-femoral artery using a proximal sartorius muscle flap: flap preparation (A) and final appearance after coverage (B).
On the seventh postoperative day, the patient was discharged in good general condition, with no evidence of infection, palpable right femoral pulses, and complete remission of symptoms. At the 6-month follow-up, CTA demonstrated patent femoral arteries without thrombus or right inguinal hematoma, and complete tissue healing (Figure 6).
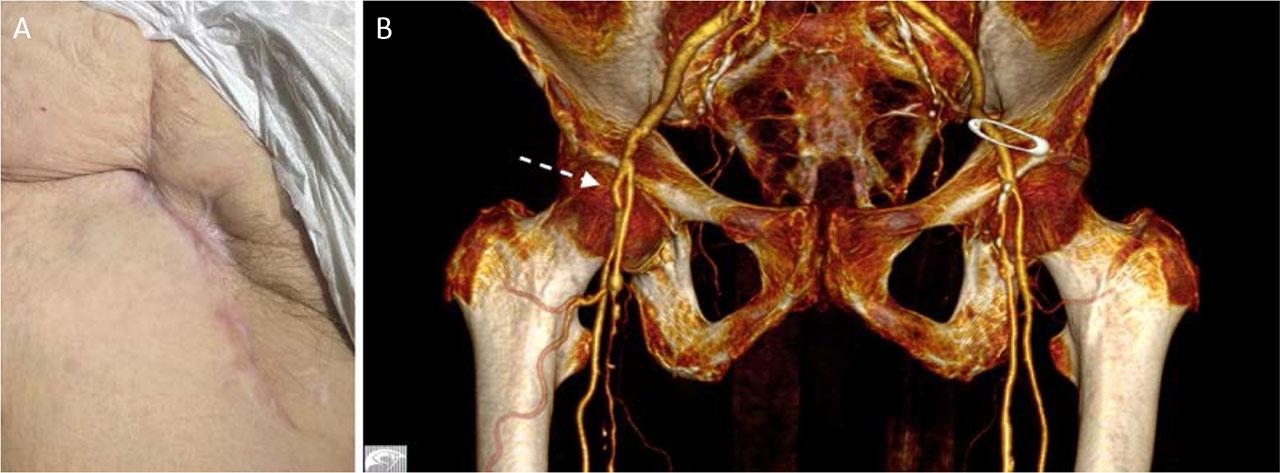
Clinical appearance (A) and 3D CTA reconstruction (B) 1 month after surgery.
Following TAVI, vascular complications are a major cause of morbidity and mortality and are also associated with increased healthcare costs and reduced quality of life. The most common complications involve the iliofemoral artery pathway.11 Graft infection can result from surgical site infection, a well-known complication of peripheral arterial reconstructive surgery. Although relatively uncommon,12 graft infection is associated with very high rates of amputation and mortality (52% and 58%, respectively), with a major impact on the outcome of revascularization procedures.12,13
In a study by Lee et al.,13 292 out of 317 patients underwent femoral endarterectomy. Groin infections occurred in 19.6% of cases: 6.3% developed deep infections extending into muscle and fascia, and 13.2% developed superficial infections. The median time to diagnosis was 17.5 days after surgery. Reported risk factors included diabetes mellitus, coronary artery disease, congestive heart failure, and chronic kidney disease, whereas current smoking was less prevalent among affected patients. Amputation and 30-day mortality rates in patients with groin infections were 11.3% and 6.5%, respectively.13 Although the current trend in the literature favors graft preservation through negative-pressure wound therapy, with or without muscle flap coverage,14,15 autologous material should be used whenever possible for lower limb revascularization. We strongly recommend the use of any available vein, such as the superficial femoral vein, to achieve favorable mid- and long-term outcomes.
Regarding the use of autologous vein grafts in arterial reconstruction after open surgery and infection of synthetic grafts or patches, the femoropopliteal vein is the most frequently used option.16,17,18,19,20,21,22 In a study by Dorsweiler et al.,16 which analyzed 19 femoral artery reconstructions utilizing femoral vein grafts, a patency rate of 87%, limb salvage rate of 93%, and a reintervention-free rate of 81% were reported. In addition, Beck et al.18 observed that smaller graft size, a history of coronary artery disease, and smoking were associated with stenosis of the femoropopliteal vein. Similar findings were documented by Daenens et al.19 and Gibbons et al.21 In the present case, due to the smaller diameters of the femoral arteries, we opted to use the great saphenous vein to fashion a bifurcated graft. Similarly, Tsunekawa et al.23 reported excellent one-year outcomes after carotid artery reconstruction with an autologous bifurcated saphenous vein graft.
This case illustrates the challenges of vascular reconstruction using a Y-shaped graft fashioned from two GSV segments in an elderly patient with multiple comorbidities and a history of infected prosthetic patches. Despite advanced age, frailty, and significant cardiovascular risk, a tailored surgical approach, including extensive debridement, autologous reconstruction, and sartorius muscle flap coverage, achieved successful short-term outcomes. At 6 months, the patient demonstrated complete wound healing, patent grafts, restored blood flow, and no signs of infection, underscoring the value of individualized vascular surgery even in high-risk elderly patients.