Forests, vital ecosystems, provide a wide range of ecosystem services, such as timber and non-timber forest products, carbon sequestration, biodiversity conservation, soil formation and nutrient cycling. All these services can be achieved from healthy forests. A healthy forest refers to the overall condition of the forest and its ability to support ecological and economic functions over time (Trumbore et al. 2015). Healthy forests are not only free of diseases, pest infestations, alien invasions and pollution, but also resistant to a wide variety of stresses and diseases (Warren 2007; Linnakoski et al. 2019).
Currently, climate change events, pollution and invasions of alien species have increased the risk of diseases in forests, as these factors weaken plants, making them more susceptible to diseases and pests (Solomou et al. 2019; Burdon and Zhan 2020; Panzavolta et al. 2021). Various types of diseases caused by fungi, bacteria, viruses, nematodes, insects and other pests have become more severe with increasing intensities of the abovementioned factors; therefore, forest health is deteriorating and productivity has been reduced (Guégan et al. 2020; El-Sayed and Kamel 2020; Paseka et al. 2020).
Nepal is a country with diverse forest ecosystems from tropical lowlands to subalpine regions that provide a range of ecosystem services and support livelihoods for many communities (Lamsal et al. 2018). As climate change events, pollution and alien plant invasion have been serious problems, Nepal’s forests are facing a range of direct negative impacts from these factors (Thapa et al. 2017, 2020; Shrestha and Shrestha 2021; Charmakar et al. 2022; Wani et al. 2023). In addition, serious infestation by pathogens has been reported throughout the country (Malla and Pokharel 2018). Forest disease is an important and complex area of research that requires interdisciplinary approaches to understand the causes and consequences of diseases in forests, but it is often a neglected area of research in Nepal. This study was carried out with the aim of assessing highly problematic diseases of tree species in Nepal. The study will have significance in understanding and will be useful for future planning of the management of forest pathogens and preserving forest health in the country.
In this study, a total of seven districts (Taplejung, Nawalpur, Tanahun, Lamjung, Dang, Dadheldhura and Darchula) of eastern, central and western Nepal were visited (Fig. 1, Tab. 1). Diseased plant samples were collected during August–September 2020, and the samples were packaged in sterile paper bags with labelling of latitude, longitude, altitude and collection number. The samples were kept in icebox and transported to the Plant Pathology Laboratory of Forest Research and Training Center, Ministry of Forestry, Babarmahal, Kathmandu, Nepal. The samples were stored in refrigerator at 4°C for further analysis. All the host specimens were also identified using standard literature such as Shrestha et al. (2022).
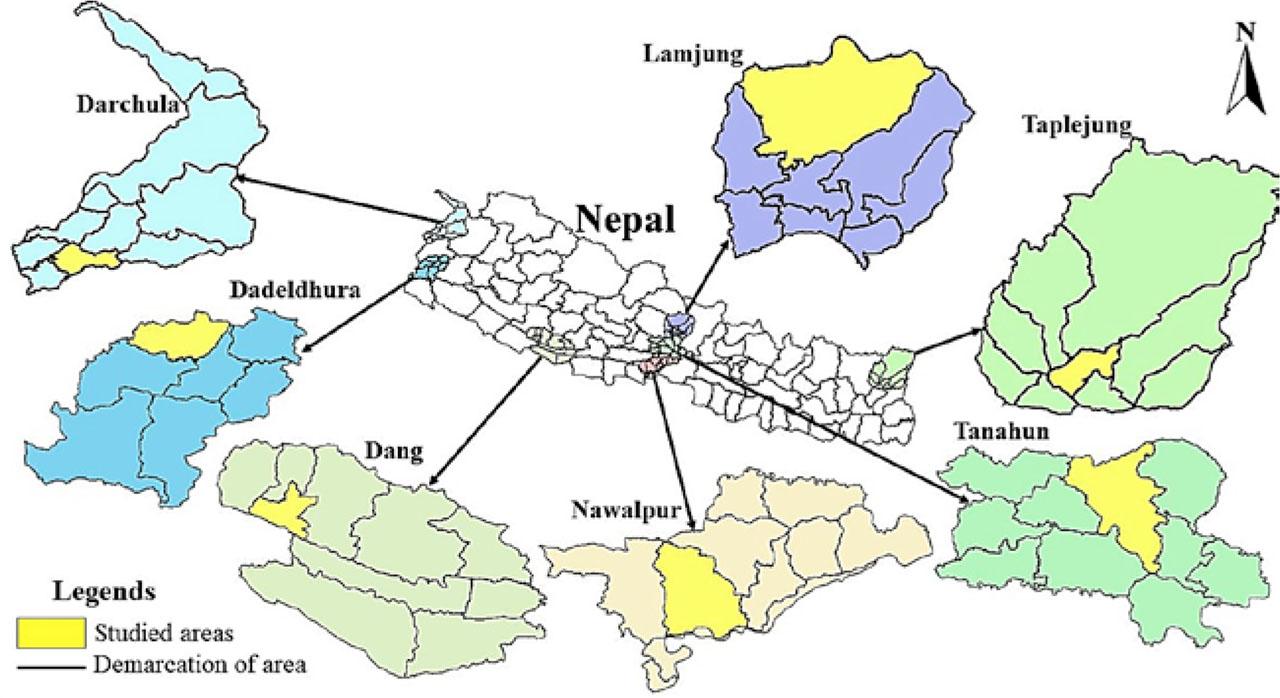
Map showing sample collection sites
Details of sample collection sites in Nepal
| S. No. | Name of host plant | Diseased parts | Location | Elevation (m) | Forest | Municipality and district |
|---|---|---|---|---|---|---|
| 1 | Abies spectabilis | needles | 27.422°N | 3413 | Mayam Patal CF | Phungling Municipality, Taplejung |
| 2 | Acer laevigatum | leaves | 27.365°N | 2642 | Deurali-Bhitri CF | |
| 3 | Rhododendron arboretum | leaves | 27.427°N | 364 | Mayam Patal CF | |
| 4 | Alnus nepalensis | leaves | 28.293°N | 901 | Tatopani CF | Marsyangdi RM, Lamjung |
| 5 | Alnus nepalensis | trunk | 29.336°N | 1639 | Ugratara CF | Ajaymeru RM, Dadheldhura |
| 6 | Eucalyptus camaldulensis | leaves | 28.095°N | 561 | Eucalyptus PF | Dangisharan RM, Dang |
| 7 | Pinus roxburghii | needles | 29.698°N | 1416 | Shalya Shikhar CF | Shailyashikhar Municipality, Darchula |
| 8 | Shorea robusta | stem | 27.622°N | 197 | Sal SF | Madhyabindu Municipality, Nawalpur |
| 9 | Tectona grandis | leaves | 28.095°N | 561 | Teak PF | Dangisharan RM, Dang |
| 10 | Toona ciliata | leaves | 27.989°N | 487 | Gairigau CF | Vyas Municipality, Tanahun |
Note. CF: Community Forest, PF: Planted Forest, SF: Scientific Forest, RM: Rural Municipality
The diseased samples were washed thoroughly, surface sterilised with 0.2% sodium hypochlorite and again washed with sterile distilled water. Then, small fragments (5 × 5 mm) of infected portions were placed on Petri plates containing potato dextrose agar media supplemented with 100 mg/L streptomycin sulphate. The plates were incubated at 25±2°C for 5–7 days (Johnston and Booth 1983). The hyphal tips from each developing colony’s margin were subcultured as pure isolates. Non-culturable pathogens were observed by cutting the leaf sections through the infected portions.
Cultured pathogens were observed using an OPTIKA B-380 LED light microscope. In addition, measurements of isolated fungi were taken using OPTIKA Microscopy software. The pathogens were identified based on the morphological characteristics of colonies, hyphae, fruiting bodies and reproductive structures, following Gilman (1957), Barnett and Hunter (1998), Braun and Cook (2012) and Campbell and Johnson (2013). Taxonomic verification and assigning the nomenclature of pathogens and their current names were performed based on Mycobank (https://www.mycobank.org/).
Altogether, 10 pathogenic fungi from different tree hosts were isolated and identified. Among them, eight pathogens were identified from foliar diseases and two were identified from stem and trunk diseases. The foliar pathogens were Neonectria neomacrospora from the host Abies spectabilis, Aureobasidium apocryptum from Acer laevigatum and Golovinomyces cichoracearum from Alnus nepalensis. Similarly, Calonectria reteaudii was isolated from the leaves of Eucalyptus camaldulensis, Dothistroma septosporum from Pinus roxburghii needles and Calonectria indusiata from Rhododendron arboreum. The pathogens Olivea tectonae and Rhytisma acerinum were isolated from the host trees Tectona grandis and Toona ciliata, respectively. Meanwhile, Phytophthora alni subsp. Alni and Nectria sp. were isolated from the stems of Alnus nepalensis and Shorea robusta, respectively (Annex I). The diseases caused by these pathogens, symptomatology and identifying characteristics are given in Table 2.
Host plants, disease name, pathogens, symptomatology and identifying characters
| S. No. | Host plant | Disease | Pathogen identified | Symptomatology | Identifying characters | |
|---|---|---|---|---|---|---|
| colony colour | vegetative and reproductive characters | |||||
| 1. | Abies spectabilis | needle cast | Neonectria neomacrospora | infected needles were light brown to dark brown; young needles were uninfected | white | hyphae: septate; microconidia: size 10.3–13.6 × 3.7 µm, multicellular, cylindrical or elongated, slightly curved and up to 93.11 µm |
| 2. | Acer laevigatum | leaf anthracnose | Aureobasidium apocryptum | light brown spots and large necrotic patches on leaves, some of the leaves were entirely covered by necrotic patches | greyish white | hyphae: septate; small acervuli erupted through the epidermis of leaf; conidia: size 6.5–10.5 × 3–4.5 µm, hyaline, non-septate borne on short, broad conidiophores |
| 3. | Alnus nepalensis | powdery mildew | Golovinomyces cichoracearum | white powdery spores on leaves, chlorosis and premature dropping | white | hyphae: hyaline and smooth (3.5–6.8 μm wide); conidia: hyaline, cylindrical or ellipsoidal, size 28.7–18.7 µm, developed at the apex of conidiophores |
| 4. | Alnus nepalensis | bleeding canker | Phytophthora alni subsp. alni | the infected trunk was oozing reddish brown fluid from cracks, dieback symptom was also reported | light brown, white at maturity | hyphae: aseptate; sporangiophores: slender, unbranched; zoosporangia: size 35–65 × 24–50 µm, ovoid or pear shape |
| 5. | Eucalyptus camaldulensis | leaf blight | Calonectria reteaudii | grey water-soaked spots, necrotic patches, some leaves were defoliated | light grey | hyphae: septate; conidia: size 65–85 × 5–6 µm, straight or cylindrical, round at both ends |
| 6. | Pinus roxburghii | pinus needle cast | Dothistroma septosporum | yellowish-tan spots on needles, reddish brown bands around the needle, premature death and drop of infected needles | white with abundant aerial hyphae | Conidiophores: numerous, hyaline; conidia: septate, hyaline, straight or slightly curved, size 16.4–23.6 × 3 µm |
| 7. | Rhododendron arboreum | leaf spot | Calonectria indusiata | dark brown spots on leaves, somewhere the spots covered whole leaves, premature defoliation | white with abundant aerial hyphae. | hyphae: septate; conidiophores: penicillate, two to six phialides; conidia: size 60–70 × 4–6 μm, straight or cylindrical, round at both ends, septate |
| 8. | Shorea robusta | stem canker | Nectria sp. | oval to elongated, brown to reddish lesions on the bark of trunk and branches, oozing sap with foul smell | cottony with white with aerial hyphae | Hyphae: septate; conidiophores: verticillate, one to three branched; conidia: ellipsoidal to cylindrical or slightly curved, hyaline, smooth, rounded at both ends |
| 9. | Tectona grandis | leaf rust | Olivea tectonae | small, angular, brown to grey necrotic areas on upper leaf surface, necrosis due to coalesced lesions, powdery yellowish rusts | not cultured | Uredospores: powdery, orange coloured, ovoid and echinulated, size 18–23 × 17.5–24.8 μm |
| 10. | Toona ciliata | tar spot | Rhytisma acerinum | small, superficial black tar-like spots | greyish brown | ascomata: 40–120 μm wide with asci and paraphyses; ascospores: ellipsoidal, blunt at both ends, size 40–70 × 9–10 μm |
The distribution of fungal pathogens is shown in Figure 2. The pathogens Neonectria neomacrospora, Aureobasidium apocryptum and Calonectria indusiata were reported from the eastern part of Nepal (Koshi Province). From the western part (Gandaki Province), the pathogens Golovinomyces cichoracearum, Nectria sp. and Rhytisma acerinum were reported. Similarly, two of the pathogens (Calonectria reteaudii and Olivea tectonae) were found in western Nepal (Lumbini Province) and Dothistroma septosporum and Phytophthora alni subsp. alni were reported from the far western part of Nepal (Sudurpashchim Province) (Fig. 2).
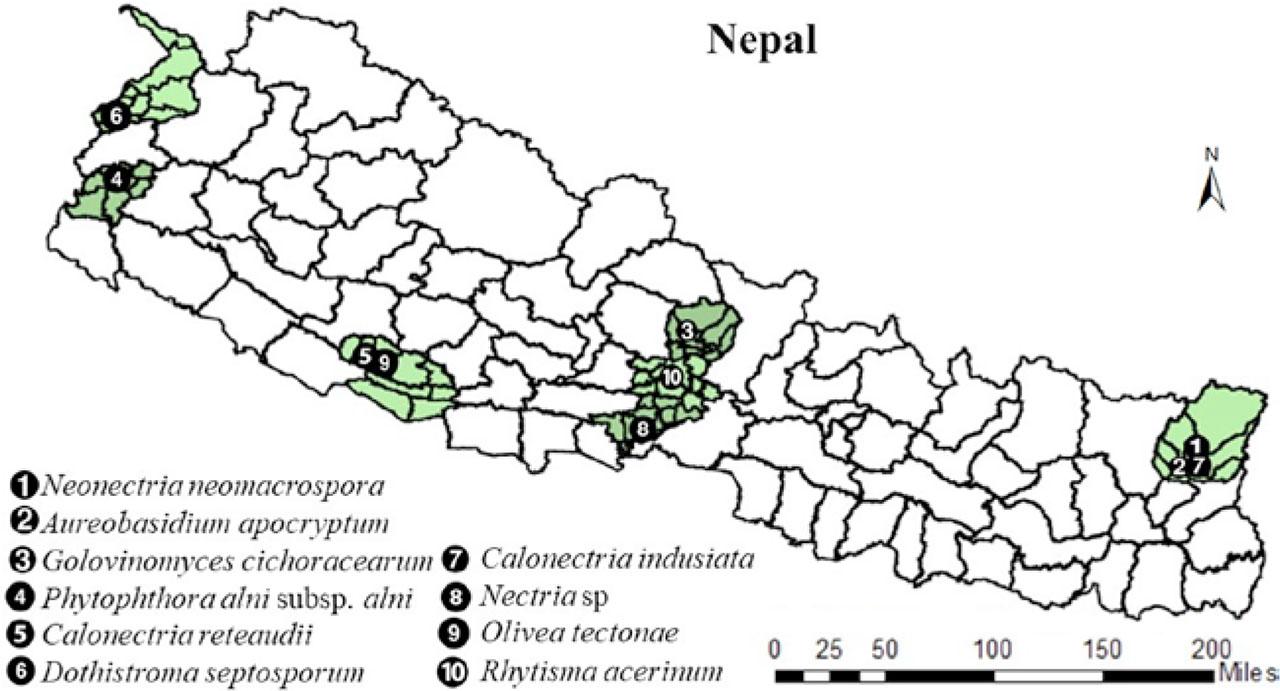
Distribution of potential invasive fungal pathogens in Nepal
The fungal pathogen Neonectria neomacrospora was reported in Norway, Denmark and England from 2008 to 2015, causing stem canker and dieback disease in different species of Abies (Nielsen et al. 2017). Due to the re-emergence of this pathogen causing different symptoms on shoots, stems, branches, needles and cones, it was identified as an aggressive pathogen of Abies and was included in the European and Mediterranean Plant Protection Organization (EPPO) Alert list (González et al. 2021). In Nepal, the pathogen causing needle cast disease was reported in Abies spectabilis by González et al. (2021).
Another pathogen, Aureobasidium apocryptum, was reported in the leaves of Acer sp. from Canada (Creelman 1965) and North America (Vujanovic and Brisson 2002). Bamadhaj et al. (2015) reported this pathogen as a potential invasive pathogen. The incidence of this pathogen in Nepal might be a new record from Nepal. Literature related to the pathogen Golovinomyces cichoracearum in Alnus nepalensis was not found. Instead, Erysiphe penicillata and Oidium sp. were reported in Alnus glutinosa (Mieslerová et al. 2020) and Alnus nepalensis (Srivastava and Verma 1990) from the Czech Republic and Sikkim (India), respectively. Another species of Golovinomyces was reported as an invasive pathogen by Félix-Gastelum et al. (2019) in Mexico.
A destructive pathogen Phytophthora from Alnus trees was found in 1993 in the United Kingdom and was later confirmed to exist in various regions of Europe (Brasier et al. 1995). The pathogen was found to be locally very damaging and spreading with a threat to both natural and managed alder populations in Europe, Asia and North America (Redondo et al. 2015; Jung and Blaschke 2004; Sims et al. 2015). In Vietnam, Phytophthora sp. close to Phytophthora tropicalis was reported by Jung et al. (2020) from Alnus nepalensis causing bleeding canker and was designated Phytophthora sp. tropicalis-like 2. The pathogens isolated in this study were identified as Phytophthora alni subsp. alni on the basis of the morphological characteristics of the pathogen as described by Hansen (2012).
Calonectria reteaudii (Anamorph: Cylindrocladium reteaudii) is a well-recognised pathogen responsible for the decline of Eucalyptus trees (Bose et al. 2022). In Vietnam and Indonesia, 10 undescribed new species of Calonectria were reported in Eucalyptus and Acacia (Pham et al. 2019). Members of this complex are widespread worldwide in natural and Eucalyptus plantations and have recently been reported from India, Malaysia, southern China, Brazil and Thailand (Jessadarom et al. 2018; Wu and Chen 2021; Pham et al. 2022; Bose et al. 2022; Sanchez-Gonzalez et al. 2022). No previous literature was found regarding this pathogen in Nepal, and therefore, it is expected that this is a new report from the country.
Dothistroma needle blight (DNB) is one of the most serious foliar diseases of Pinus spp. in many countries (Drenkhan et al. 2016). Dothistroma septosporum is the root cause of DNB, together with Dothistroma pini (Barnes et al. 2004). The disease symptoms for both pathogens are necrotic lesions on the needle, frequently accompanied by red bands, and early needle loss (Barnes et al. 2004). The disease caused by Dothistroma septosporum (reported from this study) in Pinus spp. has gained worldwide attention because of the terrible damage to pine plantations (Bakshi and Singh 1968; Barnes et al. 2022). Another species, Calonectria, is frequently associated with a variety of disease symptoms, such as leaf spot, stem rot, canker, blight, root and pod rot (Crous 2006). Calonectria indusiata was first reported in Rhododendron from Florida, USA (Crous et al. 2000) and described by Crous (2002) as the most prevalent canker disease pathogen.
Nectria canker is common in hardwood tree species from the temperate regions of the northern hemisphere (Lortie 1974; Yang et al. 2018). In the present research, canker in Shorea robusta was found to be caused by Nectria sp., but no literature was found supporting this finding. The pathogen Olivea tectonae is the major rust fungus in the tree species Tectona grandis, causing premature defoliation and reduction of the expected growth rates from nursery to adult plants (Cabral et al. 2010; Pathak et al. 2015; Koffi et al. 2018). This fungus is categorised as an invasive pathogen in the Oceania region, and the disease has also been reported throughout Asia (CABI/EPPO 2015; EPPO 2022).
Tar spot is another common plant disease caused by different pathogenic fungi depending on the type of host plant. Among the different causal agents, Rhytisma acerinum is the major agent that chiefly causes the disease in Mapple (Hudler et al. 1987), but it was also reported in Toona ciliata in the present research work, which was first reported in India (Chandel and Kumar 2017). The majority of pathogens were previously documented from different regions of the world (Farr et al. 2021); however, there was no prior record of these pathogens in Nepal.
Nepal’s forests serve as an ecological bridge between South Asian and East Asian biomes, so pathogens discovered here pose immediate cross-border threats to neighbouring India, China and Bhutan. As a sentinel site, Nepal’s initial reports of these globally devastating infections serve as an early warning system for future continent-scale shifts, illustrating how invasive fungi might exploit new hosts and habitats. Climate change threatens to broaden disease ranges; rising temperatures may allow subtropical dangers to spread to temperate zones around the world, transforming localised infections into international crises (Gullino et al. 2022; Kumar and Mukhopadhyay 2025). Biocontrol presents an effective alternative to chemical pesticides and fertilisers in fighting forest pathogens, though more research is needed to advance biological control agents against such invasive threats (Balla et al. 2021).
Overall, a total of 10 pathogenic fungi that are potentially invasive pathogens from different host trees in Nepal were isolated and identified. Most of the pathogens caused foliar disease with different symptomatology, including blight, spots, powdery mildew, etc. The pathogens were prevalent in community-managed and planted forests. The prevalence of these pathogens was mostly on timber trees, such as Shorea robusta, Abies spectabilis and Tectona grandis. These findings constitute the first documented occurrences of these phytopathogens within Nepal, indicating the emergence of invasive forest diseases in the region. Critically, the detection of globally destructive agents such as Phytophthora alni subsp. alni (on Shorea robusta) and Dothistroma septosporum (on Pinus roxburghii), underscores their potential as transboundary phytosanitary threats to ecologically pivotal tree species. Consequently, comprehensive longitudinal surveillance and expanded research across Nepal’s forest ecosystems are imperative to map pathogen distribution, assess invasion pathways and implement pre-emptive management strategies to mitigate further dissemination.