Figure 1.

Figure 2.
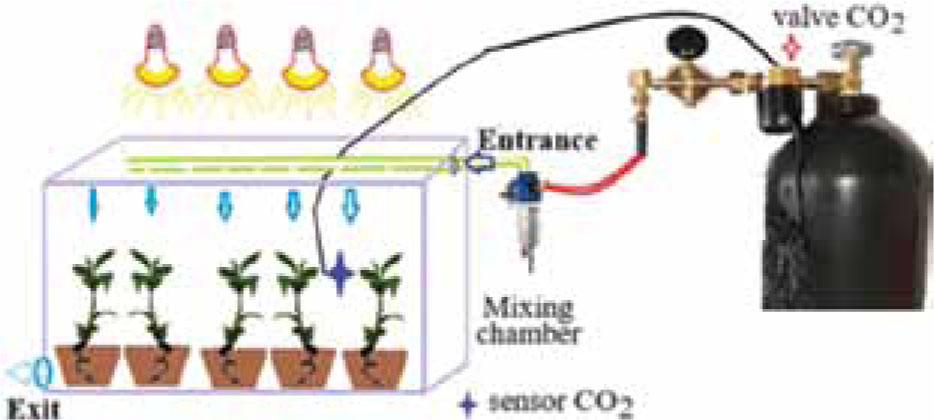
Figure 3.
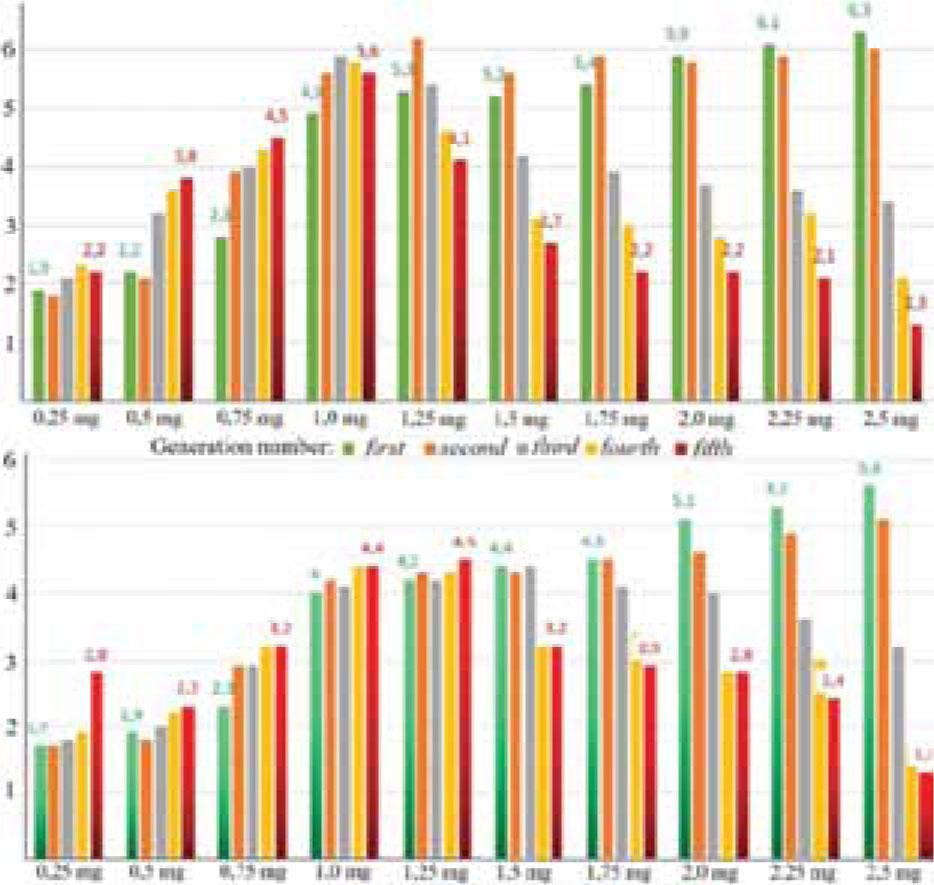
Figure 4.

Figure 5.
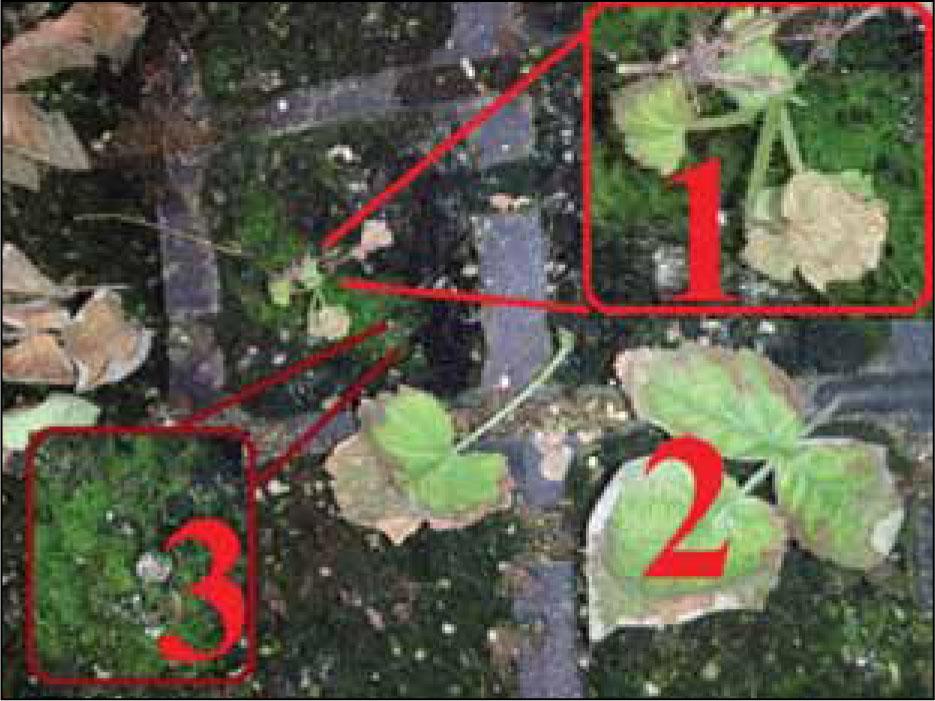
Figure 6.

Figure 7.

Figure 8.

Figure 9.

Figure 10.

Figure 11.
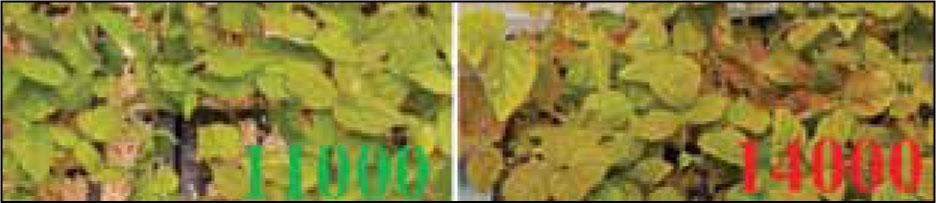
Figure 12.

Figure 13.

Duration of plant regeneration in vitro from different types of primary explants, days
| Type of explant | Medium | ||
|---|---|---|---|
| MS | M1 | MK | |
| Meristem | 63 ± 7.2 | 57 ± 8.1 | 61 ± 7.0 |
| Bud | 39 ± 3.9 | 33 ± 4.8 | 30 ± 4.1 |
| Shoot stem | 34 ± 4.7 | 32 ± 4.2 | 30 ± 3.8 |
Peculiarities of adaptation of regenerants depending on the basis of the substrate
| Indicator | The basis of the substrate | |
|---|---|---|
| Peat | Perlite | |
| Survival, % | 73.3 ± 6.0 | 89.3 ± 6.3 |
| Height of regenerants on the 30th day, mm | 76.5 ± 6.0 | 67.1 ± 5.3 |
| Inhabitation by insects, pieces/container | 23.1 ± 6.4 | 4.0 ± 1.7 |
The influence of the nutrient medium on the rate of raspberry multiplication in vitro (BAP 1_0 mg/l, fifth passage)
| Indicator | Medium | ||
|---|---|---|---|
| MS | MK | M1 | |
| Multiplication coefficient | 4.7 ± 0.3 | 2.1 ± 0.3 | 5.6 ± 0.3 |
| Regeneration period, days | 32.0 ± 4.4 | 49.0 ± 4.9 | 24.0 ± 3.2 |
Effectiveness of controlling the quantity of insects in humid chambers
| Number of insects | Control | Standard | Insectivorous plants | ||
|---|---|---|---|---|---|
| Drosera | Dionaea | Nepenthes | |||
| Peat substrate | |||||
| Beginning | 9±3.2 | 10±3.0 | 9±2.9 | 11±4.2 | 8±2.8 |
| End | 27±4.9 | 7±1.8 | 3±0.9 | 19±4.0 | 23±5.7 |
| Perlite substrate | |||||
| Beginning | 3±0.9 | 3±0.6 | 4±0.6 | 3±0.4 | 4±1.1 |
| End | 11±2.8 | 5±1.2 | 1±0.2 | 9±1.9 | 8±2.9 |
The influence of the type of primary explants on the number of transfers and the yield of regenerants in vitro, medium M1
| Type of explant | Number of transfers, pieces | Yield of regenerants from primary explants, % |
|---|---|---|
| Meristem | 2.7±0.4 | 2.3±1.1 |
| Bud | 1.9±0.3 | 18.4±3.6 |
| Shoot stem | 1.5±0.3 | 54.3±4.1 |
The composition of nutrient media
| Component | Quantity, mg/l | ||
|---|---|---|---|
| MS | M1 | MK | |
| NH4NO3 | 1650.00 | 1250 | 417.00 |
| KNO3 | 1900.00 | 1100 | 367.00 |
| MgSO4 × 7H2O | 370.00 | 770 | 257.00 |
| KH2PO4 | 170.00 | 970 | 324.00 |
| Ca(NO3)2 × 4H2O | – | 440 | 293.00 |
| CaCl2 × 2H2O | 440.00 | – | – |
| FeSO4 × 7H2O | 27.80 | – | 18.54 |
| Na2MoO4 × 2H2O | 37.30 | – | 24.70 |
| Ferrilene 4.8 Orto–Orto | – | 183.4 | – |
| H3BO3 | 6.2 | ||
| MnSO4 × H2O | 22.3 | ||
| CoCl2 × 6H2O | 0.025 | ||
| CuSO4 × H2O | 0.025 | ||
| ZnSO4 × 7H2O | 8.6 | ||
| Na2MoO4 × 2H2O | 0.25 | ||
| KJ | 0.83 | ||
| Thiamine-HCl | 1.6 | ||
| Pyridoxine-HCl | 0.5 | ||
| Vitamin C | 2.0 | ||
| Nicotinic acid | 1.0 | ||
| Mesoinosit | 100 | ||
| Glycine | 0.5 | ||
| Adenine | 0.2 | ||
| Saccharose | 30,000 | ||
| Agar | 7,000 | ||
| pH 5.6 | |||
The influence of cultivation of donor plants during five passages on media with different concentrations of cytokinins on the rhizogenesis of regenerants
| Indicator | Cytokinin, mg/l | |||
|---|---|---|---|---|
| BAP 0.25 | BAP 1.0 | Kinetin 0.25 | Kinetin 1.0 | |
| Beginning of root formation, days | 19.0 ± 3.2 | 24.0 ± 3.7 | 13.0 ± 2.4 | 16.0 ± 2.9 |
| The length of the root system on the 30th day of cultivation, mm | 9.3 ± 3.0 | 4.2 ± 0.9 | 16.4 ± 3.1 | 14.1 ± 3.9 |
| The number of roots on the 30th day of cultivation | 9.2 ± 2.8 | 7.4 ± 2.2 | 7.3 ± 2.4 | 5.1 ± 1.8 |
The length of the raspberry root system ex vitro, mm
| Conditions | Cultivation day | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 30 | |
| Wet chamber | – | 3±1.2 | 11±5.4 | 59±8.8 |
| Bioreactor | 27±3.2 | 68±7.0 | 103±9.1 | 124±11.0 |