Medullary carcinoma (MC) of the colon is one of the rarest types of colon adenocarcinoma which accounts for 0.03% of all colorectal carcinomas[1]. In a retrospective study conducted by Jabbal et al. in colon cancer patients from the National Cancer Database (NCDB) from 2004 to 2018, they identified 1,041,753 patients with colon cancer, among which 2709 patients had MC[2]. MC tumors are poorly differentiated, have high frequency of microsatellite instability-high (MSI-H; 82.4%), and are more prevalent in older patients and women[2]. Given the rarity of the tumor, no specific standard guidelines exist to guide treatments and prognosis remains unclear. MC of colon is usually presented as stages II and III, and the prognosis for patients with stage IV MC of colon is poor, with a median survival time of only 10 months[3]. More cases are being diagnosed in the last decade due to better diagnostic approaches[4]. Here, we report a rare case of elderly male diagnosed with stage 2 MC of the colon and discuss its prognosis and treatment implications.
A 82-year-old Caucasian male with no significant past medical history presented to the hospital with abdominal pain, 7/10, gradual in onset for the past 2 months with last bowel movement 2 days ago. Computed tomography (CT) of the chest, abdomen, and pelvis on admission, as presented in Figure 1, showed thickening of the ascending colon concerning malignancy. He underwent colonoscopy, which showed a tumor in the ascending colon, with several adjacent enlarged lymph nodes (LNs) representing extension of the disease, with no acute findings in the chest. He later underwent right hemicolectomy, which showed poorly differentiated carcinoma, composed of sheets and cords of malignancy, abundant eosinophilic cytoplasm, and nuclei with vesicular chromatin and prominent nucleoli. There were some areas suggestive of poorly formed glands associated with brisk inflammatory infiltrate mainly composed of lymphoplasmacytic infiltrate at the base of the tumor as well as intercalating between tumor cells as described in Figures 2 and 3. Immunohistochemistry (IHC) stains showed patchy positivity for CDX2 and rare tumor cells positive for CK7 and negative for CK20, p40, synaptophysin, and chromogranin. There was loss of expression of MLH1 and PMS2. All the above features are suggestive of MC with invasion into pericolonic tissue, 0/44 LNs involved, negative margins, with no lymphovascular invasion (LVI) or perineural invasion (PNI) and deficiency in mismatch repair protein (dMMR) (loss of MLH1, PMS2 expression) noted. Final stage pT3N0 (Stage 2) was diagnosed. With the given tumor being pT3 and MSI-H, active surveillance was recommended with no adjuvant therapy. He is currently doing well with no signs of disease recurrence.
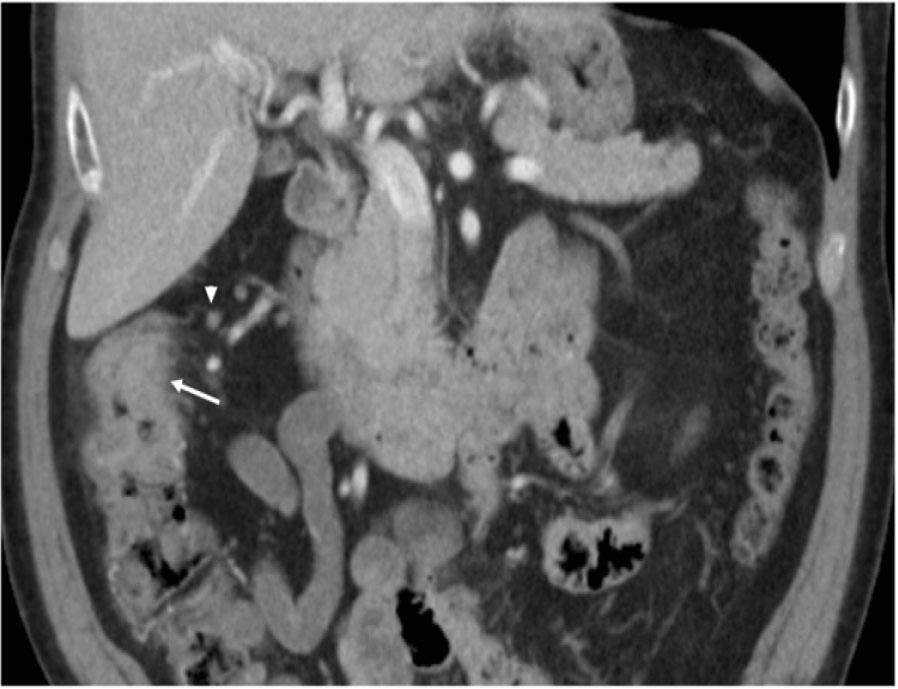
Circumferential thickening and heterogenous attenuation of the ascending colon just proximal to the level of the hepatic flexure.

Higher magnification reveals poorly differentiated cells with medium to large-sized vesicular nuclei, nucleoli, and moderate eosinophilic cytoplasm. The larger carcinomatous cells are interspersed by the smaller tumor infiltrating lymphocytes.
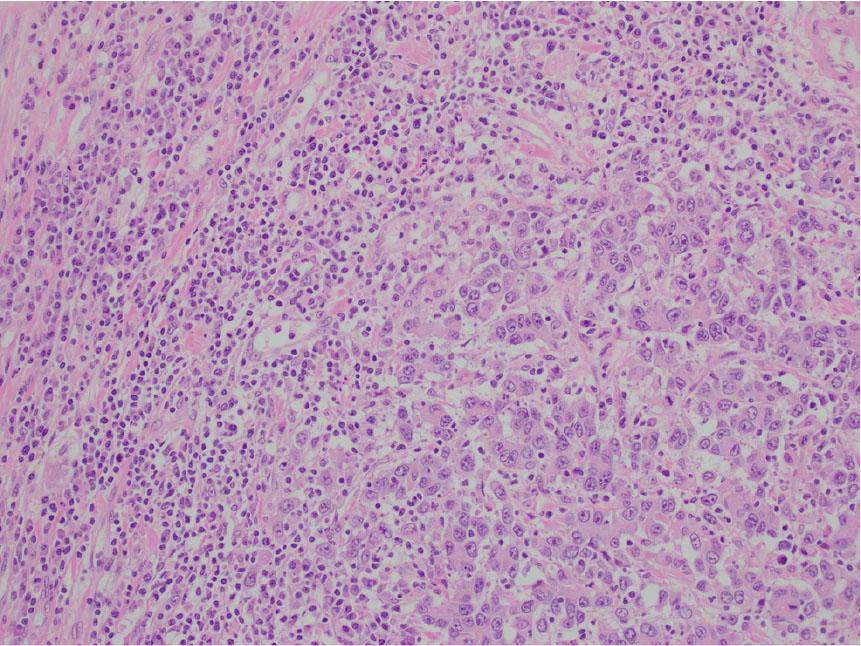
The medullary carcinoma is composed of poorly to undifferentiated cells in a vaguely trabecular to solid pattern. It lacks the typical glandular formation and mucin content seen in traditional colonic adenocarcinomas. The carcinoma has a circumscribed, pushing border, with a brisk intraepithelial and peritumoral lymphocytic infiltration.
MC is one of the rare types of colon adenocarcinoma, and lack of understanding of these tumors for many years has resulted in incorrect diagnosis[1]. It was first described in the 1990s with an approximate incidence of 0.03%[2]. In a retrospective study conducted by Scott et al. from 1997 to 2018, they found that only one-third of MC patients were correctly identified[3]. Comprehensive pathological examination is important along with IHC staining for accurate diagnosis of MC of the colon[4]. Tumor cells in MC are poorly differentiated, arranged in sheets, highly proliferative with abundant cytoplasm and prominent nucleoli, and lack glandular formation with abundant lymphocyte infiltration[1]. Usually, differential diagnosis of poorly differentiated adenocarcinoma associated with dense lymphoid infiltrate includes two subtypes, which are lymphoepithelioma-like carcinoma (LELC) associated with Epstein–Barr virus (EBV) infection and MC[5]. MC is noted to have a well-defined border with peritumoral inflammation and is frequently noted to be MSI-H, unlike LELC which is formed by small clusters of cells with no syncytial growth pattern[5]. Apart from relying entirely on histology, it is recommended to determine EBV and MSI status to help distinguish between these tumors[5].
Since MC presents as poorly differentiated carcinoma, it is important to differentiate it from pancreatic ductal adenocarcinoma (PDA) or undifferentiated adenocarcinoma (UDA), where IHC plays a major role apart from histology[6]. Usually, PDA cells show severe variation in sizes and shapes of nuclei and chromatin variation compared to MC cells[6], whereas MC cells show intense peritumoral lymphoplasmacytic infiltration compared to PDA cells[6]. PDA cells exhibit more areas of gland formation compared to MC cells[6].
In a retrospective study conducted by Winn et al., the authors found that MC cells significantly express calretinin (73% MC vs. 12% in PDA) with significantly reduced expression of MLH1[6]. Staining pattern of Trefoil Factor 3 (TFF3), Transmembrane Glycoprotein mucin 1 (MUC1), and transmembrane glycoprotein mucin 2 (MUC2) in MC showed diffuse cytoplasmic pattern compared to membranous pattern, which is usually found in normal colon epithelium[6]. They also noted that cells with calretinin-positive phenotype with markers for CDX2 and MLH-1 negative have 82% positive predictive value in diagnosing MC[6]. In addition, presence of BRAF V600E mutation and ARID1A expression loss can also aid in diagnosis of MC[7].
Blakely et al. analyzed data from SEER (2002–2017) and NCDB program (2010–2016) to better understand the characteristics and outcomes of MC in the context of current colon cancer management[8]. From 2002 to 2017, the age-adjusted incidence rates of MC increased by 23.8%, whereas the incidence rates of age adjusted adenocarcinoma declined[8]. Left-sided tumors were associated with worse survival compared to right-sided tumors.
MC usually presents as stages II and III, and surgery remains the mainstay of treatment[4]. Adjuvant therapy in resected stage 2 colon cancer like in our patient is based on T stage and high risk factors such as the number of LNs examined, LVI, tumor perforation, tumor budding score, and bowel obstruction[9]. In pT4 disease and pT3 MSI stable and presence of high-risk factors, adjuvant therapy is recommended, whereas in pT3 MSI-H, irrespective of high-risk factors, adjuvant therapy is not recommended[9]. Treatment of pT4 MC tumors can be challenging as most of these tumors are MSI-H, and single agent 5-fluorouracil is not beneficial and addition of oxaliplatin might be required[9,10]. As mentioned above, most of these patients are elderly and addition of oxaliplatin in this age group can cause more treatment-related complications. In an analysis conducted by Friedman et al., the authors found that MC cells have upregulation of more immunoregulatory genes compared to other microsatellite unstable tumors[11]. Tumor microenvironment of MC also contains a higher mean of CD8+ tumor-infiltrating lymphocytes in epithelial and stromal compartments compared to microsatellite-unstable and microsatellite-stable tumors[7]. This calls for a question if T4, MSI-H tumors of MC are different from T4, MSI-H tumors of colon adenocarcinoma and if there is any role for adjuvant immunotherapy instead of chemotherapy as these tumors have significant upregulation of several immunoregulatory genes. It is also important to see if there is any role for neoadjuvant immunotherapy to improve outcomes. As we have better understanding of this disease over years, incidence is expected to rise and definitely more studies are needed to optimize standardized guidelines in this population.
MC of the colon is one of the rarest types of colon adenocarcinoma, the incidence of which has increased over the last decade. Although early stage cancer has better outcomes, survival rates of advanced cancer are poor. Clinically and pathologically, this tumor is considered different from colon adenocarcinoma with no standardized treatment options. More studies are required to develop guidelines to guide the role of immunotherapy in neoadjuvant and adjuvant settings for even better outcomes.