Figure (A)
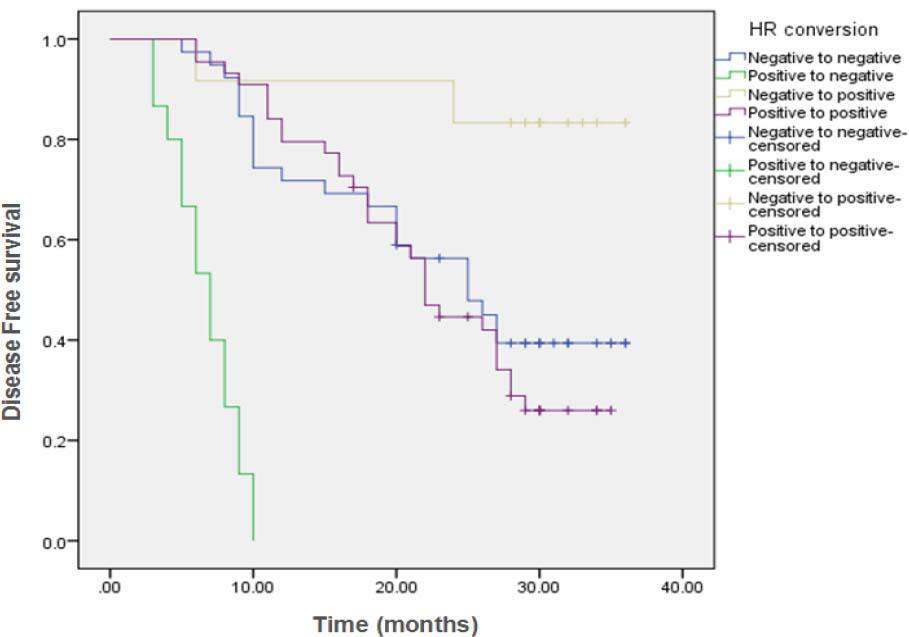
Figure (B)
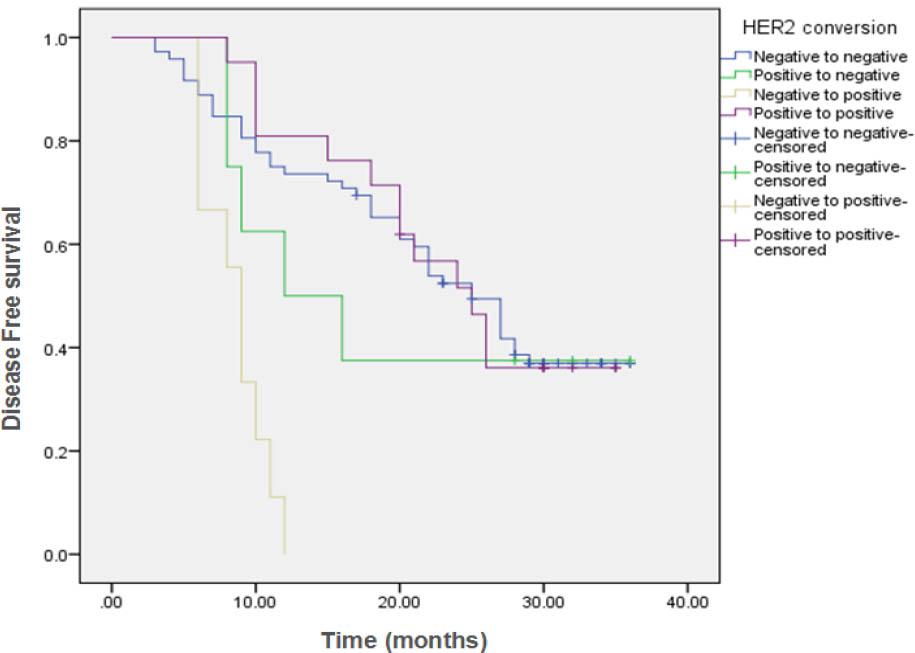
Figure (C)
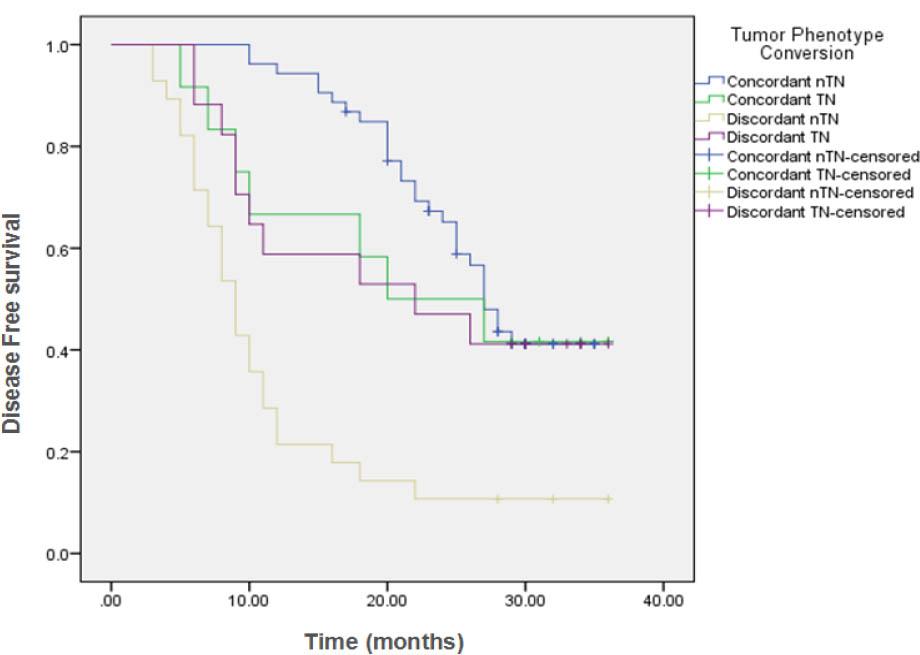
Figure (2A)
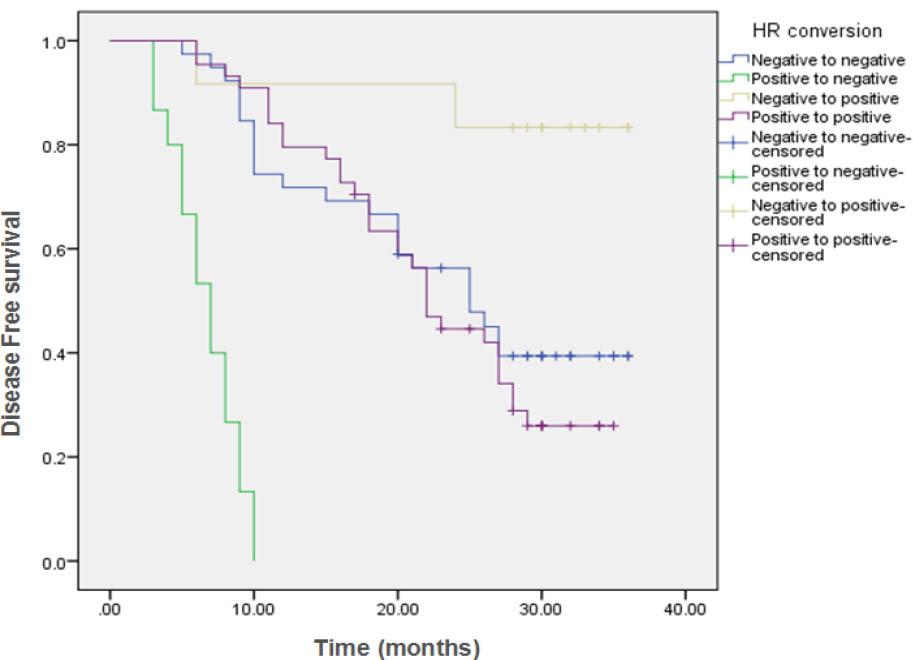
Figure (2B)
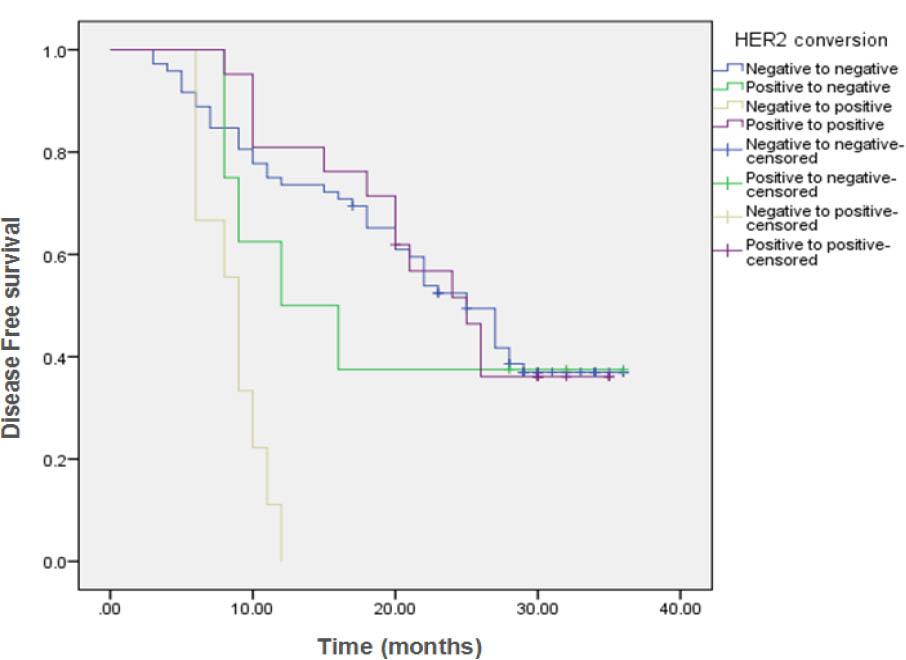
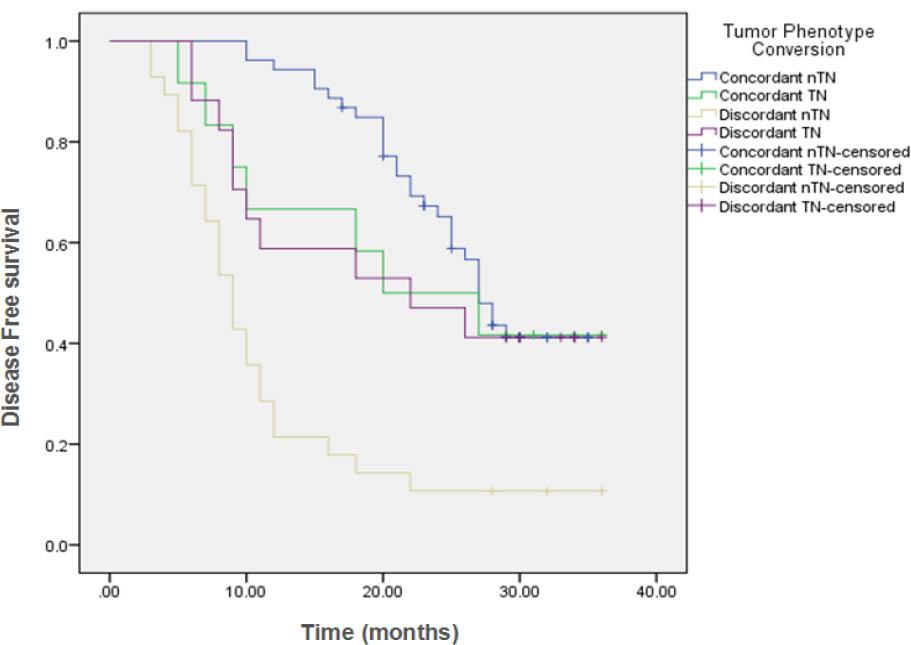
Figure (2C)
Univariate Cox regression analysis for Disease Free Survival and Overall Survival_
| Group | Total N (%) | DFS | OS | ||
|---|---|---|---|---|---|
| HazR (95% CI) | p-value | HazR (95% CI) | p-value | ||
| HR conversion | <0.001 | 0.127 | |||
| Negative to negative | 39 (35.5%) | 0.818 (0.477 – 1.405) | 0.467 | 114437.447 | 0.933 |
| Positive to negative | 15 (13.6%) | 12.606 (5.762 – 27.581) | <0.001 | 578737.963 | 0.924 |
| Negative to positive | 12 (10.9%) | 0.159 (0.038 – 0.667) | 0.012 | 67638.402 | 0.936 |
| Positive to positive | 44 (40%) | 1.000 | 1.000 | ||
| HER2 conversion | <0.001 | 0.557 | |||
| Negative to negative | 72 (65.5%) | 1.023 (0.551 – 1.901) | 0.943 | 0.381 (0.102 – 1.420) | 0.151 |
| Positive to negative | 8 (7.3%) | 1.295 (0.461 – 3.638) | 0.624 | 0.000 | 0.987 |
| Negative to positive | 9 (8.2%) | 6.104 (2.461 – 15.136) | <0.001 | 0.650 (0.072 – 5.864) | 0.701 |
| Positive to positive | 21 (19.1%) | 1.000 | 1.000 | ||
| Tumor Phenotype Conversion | <0.001 | 0.276 | |||
| Concordant nTN | 53 (48.2%) | 1.000 | 1.000 | ||
| Concordant TN | 12 (10.9%) | 1.298 (0.568 – 2.966) | 0.536 | 3.557 (0.593 – 21.328) | 0.165 |
| Discordant nTN | 28 (25.5%) | 4.548 (2.617 – 7.904) | <0.001 | 3.771 (0.823 – 17.280) | 0.087 |
| Discordant TN | 17 (15.5%) | 1.376 (0.670 – 2.826) | 0.385 | 1.100 (0.114 – 10.577) | 0.935 |
Comparison of Overall Survival (OS) in the different conversion groups_
| Group | Total N | N of events | Censored N (%) | Overall Survival (OS) | p-value† | |||
|---|---|---|---|---|---|---|---|---|
| Median | 1-year | 2-year | 3-year | |||||
| HR conversion | ||||||||
| Negative to negative | 39 | 5 | 34 (87.2%) | NR | 97.4% | 89.4% | 85.7% | <0.001 |
| Positive to negative | 15 | 4 | 11 (73.3%) | ---- | 69.1% | ---- | ---- | |
| Negative to positive | 12 | 1 | 11 (91.7%) | NR | 91.7% | 91.7% | 91.7% | |
| Positive to positive | 44 | 0 | 44 (100%) | NR | 100% | 100% | 100% | |
| HER2 conversion | ||||||||
| Negative to negative | 72 | 5 | 67 (93.1%) | NR | 94.3% | 92.7% | 92.7% | 0.365 |
| Positive to negative | 8 | 0 | 8 (100%) | NR | 100% | 100% | 100% | |
| Negative to positive | 9 | 1 | 8 (88.9%) | ---- | 88.9% | 88.9% | ---- | |
| Positive to positive | 21 | 4 | 17 (81%) | NR | 90.5% | 85.4% | 79.3% | |
| Tumor Phenotype Conversion | ||||||||
| Concordant nTN | 53 | 3 | 50 (94.3%) | NR | 98.1% | 96.2% | 93.9% | 0.219 |
| Concordant TN | 12 | 2 | 10 (83.3%) | NR | 90.9% | 81.8% | 81.8% | |
| Discordant nTN | 28 | 4 | 24 (85.7%) | ---- | 84.8% | 84.8% | ---- | |
| Discordant TN | 17 | 1 | 16 (94.1%) | NR | 94.1% | 94.1% | 94.1% | |
Patients and tumor characteristics_
| Characteristics | All patients (N=110) | |
|---|---|---|
| No. | % | |
| Age (years) | ||
| Mean±SD | 50.84 | ±10.83 |
| Median (Range) | 51 | (27 – 73) |
| <35 years | 10 | 9.1% |
| ≥35 years | 100 | 90.9% |
| Side | ||
| Right breast | 47 | 42.7% |
| Left breast | 63 | 75.3% |
| Menopause | ||
| Premenopausal | 50 | 45.5% |
| Postmenopausal | 60 | 54.5% |
| Initial Grade | ||
| Grade II | 37 | 33.6% |
| Grade III | 73 | 66.4% |
| Pathological type | ||
| IDC | 93 | 84.5% |
| ILC | 11 | 10% |
| Other | 6 | 5.5% |
| Initial T | ||
| T1 | 8 | 7.3% |
| T2 | 32 | 29.1% |
| T3 | 46 | 41.8% |
| T4 | 24 | 21.8% |
| Initial N | ||
| N1 | 30 | 27.3% |
| N2 | 50 | 45.5% |
| N3 | 30 | 27.3% |
| Initial Stage | ||
| Stage IIIA | 60 | 54.5% |
| Stage IIIB | 22 | 20% |
| Stage IIIC | 28 | 25.5% |
| Initial LVI | ||
| Negative | 46 | 41.8% |
| Positive | 57 | 51.8% |
| Unknown | 7 | 6.4% |
| Initial ER status | ||
| Negative | 45 | 40.9% |
| Positive | 65 | 59.1% |
| Initial PR status | ||
| Negative | 52 | 47.3% |
| Positive | 58 | 52.7% |
| Initial HER2 status | ||
| +1 | 64 | 58.2% |
| +2 | 23 | 20.9% |
| +3 | 23 | 20.9% |
| Initial HER2 FISH | ||
| Negative | 17 | 15.4% |
| Positive | 6 | 5.5% |
| Pre-NCT Tumor Phenotype | ||
| HER2−ve & HR+ve | 67 | 60.9% |
| HER2+ve & HR+ve | 6 | 5.5% |
| HER2+ve & HR−ve | 13 | 11.8% |
| HER2−ve & HR−ve | 24 | 21.8% |
| Neoadjuvant chemotherapy | ||
| CMF | 8 | 7.3% |
| FAC | 44 | 40% |
| FEC | 22 | 20% |
| AC-T | 27 | 24.5% |
| AC-D | 9 | 8.2% |
| Surgery | ||
| BCS | 15 | 13.6% |
| MRM | 95 | 86.4% |
| ER status at surgery | ||
| Negative | 52 | 47.3% |
| Positive | 58 | 52.7% |
| PR status at surgery | ||
| Negative | 52 | 47.3% |
| Positive | 58 | 52.7% |
| HER2 status at surgery | ||
| Negative | 8 | 7.3% |
| +1 | 57 | 51.8% |
| +2 | 20 | 18.2% |
| +3 | 25 | 22.7% |
| HER2 FISH at surgery | ||
| Negative | 16 | 14.6% |
| Positive | 4 | 3.6% |
| HR conversion | ||
| Negative to negative | 39 | 35.5% |
| Positive to positive | 44 | 40% |
| Negative to positive | 15 | 13.6% |
| Positive to negative | 12 | 10.9% |
| HER2 conversion | ||
| Negative to negative | 72 | 65.5% |
| Positive to positive | 21 | 19.1% |
| Positive to negative | 8 | 7.3% |
| Negative to positive | 9 | 8.2% |
| Tumor Phenotype Conversion | ||
| Concordant nTN | 53 | 48.2% |
| Concordant TN | 12 | 10.9% |
| Discordant nTN | 28 | 25.5% |
| Discordant TN | 17 | 15.5% |
| Adjuvant Herceptin | ||
| No | 7 | 6.4% |
| Yes | 31 | 28.1% |
| Adjuvant Hormonal | ||
| No | 39 | 35.5% |
| Yes | 71 | 64.5% |
| Radiotherapy | ||
| No | 29 | 26.4% |
| Yes | 81 | 73.6% |
| Recurrence | ||
| No recurrence | 39 | 35.5% |
| Recurrence | 71 | 64.5% |
| Mortality | ||
| Alive | 100 | 90.9% |
| Died | 10 | 9.1% |
| Follow-up duration (months) | ||
| Mean±SD | 24.11 | ±8.75 |
| Median (Range) | 27 | (7 – 36) |
Comparison of disease Free Survival (DFS) in the different conversion groups_
| Group | Total N | N of events | Censored N (%) | Disease Free Survival (DFS) | p-value† | |||
|---|---|---|---|---|---|---|---|---|
| Median | 1-year | 2-year | 3-year | |||||
| HR conversion | ||||||||
| Negative to negative | 39 | 23 | 16 (41%) | 25 months | 71.8% | 45% | 39.4% | <0.001 |
| Positive to negative | 15 | 15 | 0 (0%) | 7 months | 0% | ---- | ---- | |
| Negative to positive | 12 | 2 | 10 (83.3%) | NR | 91.7% | 83.3% | 83.3% | |
| Positive to positive | 44 | 31 | 13 (29.5%) | 22 months | 79.5% | 44.6% | 26% | |
| HER2 conversion | ||||||||
| Negative to negative | 72 | 44 | 28 (38.9%) | 25 months | 73.6% | 69.4% | 36.9% | <0.001 |
| Positive to negative | 8 | 5 | 3 (37.5%) | 12 months | 50% | 37.5% | 37.5% | |
| Negative to positive | 9 | 9 | 0 (0%) | 9 months | 0% | ---- | ---- | |
| Positive to positive | 21 | 13 | 8 (38.1%) | 25 months | 81% | 51.6% | ---- | |
| Tumor Phenotype Conversion | ||||||||
| Concordant nTN | 53 | 29 | 24 (45.3%) | 27 months | 84.9% | 65.2% | ---- | <0.001 |
| Concordant TN | 12 | 7 | 5 (41.7%) | 20 months | 66.7% | 50% | 41.7% | |
| Discordant nTN | 28 | 25 | 3 (10.7%) | 9 months | 21.4% | 14.3% | ---- | |
| Discordant TN | 17 | 10 | 7 (41.2%) | 22 months | 58.8% | 47.1% | 41.2% | |