Gestational diabetes mellitus (GDM) is defined by elevated blood sugar levels that manifest for the first time during pregnancy. This condition poses risks for both the pregnant woman and the developing fetus (Cui et al. 2023). Although many of the metabolic disturbances associated with GDM resolve postpartum, the likelihood of developing Type 2 diabetes mellitus (T2DM) later in life significantly increases (Sweeting et al. 2022). In addition, fetuses that experience hyperglycemic conditions may face an increased likelihood of diabetes onset later in life (Ray et al. 2024). Therefore, the timely identification, holistic intervention, and personalized management of GDM are essential and have become a focal point for obstetricians and endocrinologists (Yang et al. 2023a; Basil et al. 2024).
The pathogenesis of GDM remains incompletely understood. Similar to T2DM, insulin resistance is a hallmark of GDM. Increasing evidence suggests that aberrant inflammatory responses are critical determinants of insulin resistance (Milionis et al. 2024). For example, a study using a randomized controlled trial showed that injecting the anti-inflammatory cytokine interleukin (IL)-1β antibody LY2189102 subcutaneously to patients with T2DM for a duration of 12 weeks led to a significant decrease in glycated hemoglobin and fasting blood glucose level (Maiorino et al. 2017). Other studies have reported that IL-1β downregulates insulin receptor substrate-1, leading to insulin resistance (Cao et al. 2022). Moreover, extended periods of high blood sugar and elevated lipid levels lead to the production of reactive oxygen species, disrupting the equilibrium between oxidative stress and the body's antioxidant protection mechanisms. This imbalance ultimately leads to significant cellular harm and the start of various diseases (Cao et al. 2022).
Isorhynchophylline (IRN), a frequently occurring bioactive compound derived from Uncaria rhynchophylla, demonstrates various biological activities, such as reducing blood pressure, inhibiting cell proliferation, combating inflammation, and protecting nerve cells (Fu et al. 2023; Zhu et al. 2023). IRN inhibits inflammatory responses in endothelial cells and macrophages through the nuclear factor-κB/NOD-like receptor protein 3 (NF-κB/NLRP3) pathway (Wang et al. 2023a). Furthermore, IRN mitigates oxidative stress and mitochondrial impairment in paraquat-induced acute kidney injury through the regulation of toll-interacting protein expression (Zheng et al. 2021). Additionally, in an animal study, IRN has demonstrated positive impacts on conditions related to diabetes. The treatment with IRN triggers the activation of spliced X-box binding protein 1, leading to enhancement in diabetic encephalopathy (Wang et al. 2023b). Novel rhynchophylline analogs have also been developed as microvascular relaxation agents for treating diabetes-induced microvascular dysfunction.
Despite these promising findings, the role and mechanisms of IRN in GDM remain unclear. This study aims to investigate the effects and underlying mechanisms of IRN on GDM, focusing on its impact on blood glucose, insulin resistance, fetal weight, placental inflammation, oxidative stress, and the NLRP3 pathway in GDM mice. This study also aims to explore the therapeutic benefits of IRN for GDM, emphasizing its importance in enhancing the well-being of both mother and fetus through its targeted approach toward inflammation and oxidative stress pathways.
Pregnant C57BL/KsJ +/+ (wild type) and C57BLKsJ db/+ (db/+) mice (n = 6 in each group) were used to establish a GDM model. Mice without GDM served as a healthy control group to provide baseline comparisons. The db/+ mice were randomly divided into four groups: GDM, GDM + IRN (20 mg/kg), and GDM + IRN (40 mg/kg). IRN was purchased from Sigma-Aldrich (I8139). IRN was given orally once a day through gavage at dosages of 20 mg/kg or 40 mg/kg, commencing on gestational day 0 and continuing until gestational day 20. The Institutional Animal Care and Use Committee granted approval for all animal experiments conducted.
Blood glucose levels were assessed using intraperitoneal glucose tolerance tests (IPGTTs) and intraperitoneal insulin tolerance tests (IPITTs). The IPGTT measures the body's ability to metabolize glucose after an intraperitoneal glucose injection (2 g/kg body weight), while the IPITT evaluates insulin sensitivity after an intraperitoneal insulin injection (0.75 U/kg body weight). The experiments were carried out on day 10 of pregnancy, about 10 h following the final IRN administration. In the IPGTT, blood specimens were obtained via the tail vein at intervals of 0, 30, 60, and 120 min after glucose injection. In the IPITT, blood samples were collected at time points of 0, 15, 30, 60, and 120 min following insulin injection. Glucose concentrations were determined using a glucometer (OneTouch Ultra, LifeScan).
Serum and placental tissues were collected, and the levels of inflammatory cytokines tumor necrosis factor (TNF)-α, IL-6, and IL-1β were measured using enzyme-linked immunosorbent assay (ELISA) kits from Abcam (UK; ab100747, ab222503, ab100768).
The placental tissues were subjected to homogenization, followed by the assessment of oxidative stress markers such as malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH) using commercially available kits supplied by Beyotime Biotechnology (China; S0131, S0086, S0053).
On gestational day 20, pregnant mice were sacrificed, and the fetuses were collected. Litter size and the weight of each fetus were recorded.
Placental tissues were disrupted in RIPA buffer (Beyotime Biotechnology; P0013B) and then underwent homogenization. The same quantities of protein were subjected to separation via SDS-PAGE, followed by transfer onto polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked with 5% non-fat milk and incubated with primary antibodies against NLRP3 (Abcam; ab214185), cleaved caspase-1 (Abcam; ab179515), IL-1β (Abcam; ab9722), and IL-18 (Abcam; ab71495) overnight at 4°C. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (Abcam; ab6721) and visualized using an enhanced chemiluminescence (Beyotime Biotechnology; P0018).
Data are presented as mean ± SD. The average blood glucose levels, insulin sensitivity, and other parameters were compared among the groups using one-way ANOVA followed by Tukey's post hoc test. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad 8 software.
To uncover the potential effects of IRN on GDM, we first detect its effects on the blood glucose levels and insulin sensitivity of GDM mice. The structural formula of IRN was shown in Figure 1a. In particular, the results of the IPGTT indicated a notable decrease in blood glucose levels in the IRN-treated groups (20 mg/kg and 40 mg/kg) when compared with the untreated GDM group during the 120-min timeframe (Figure 1b). Similarly, the IPITT demonstrated improved insulin sensitivity in the IRN-treated groups, with blood glucose levels decreasing more effectively than in the untreated GDM group over the same period (Figure 1c). Moreover, GDM mice treated with IRN showed notable reductions in fasting blood glucose levels and serum insulin levels, along with elevated liver glycogen content in comparison to the GDM group that did not receive the treatment (Figure 1d). The results suggest that IRN has a beneficial impact on glucose metabolism and insulin sensitivity in mice with gestational diabetes.

Effect of IRN on blood glucose levels and insulin sensitivity in GDM mice. (A) Structural formula of IRN. (B) The IPGTT was performed on gestational day 10, with blood samples collected at 0, 30, 60, and 120 min post glucose injection. (C) The IPITT was conducted on the same day, with blood samples collected at 0, 15, 30, 60, and 120 min post insulin injection. (D) Bar graphs showing fasting blood glucose levels, serum insulin levels, and liver glycogen content in control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). Data are presented as mean ± SD. *p < 0.001, GDM vs. control, **p < 0.001, GDM + IRN vs. GDM. GDM, gestational diabetes mellitus; IPGTT, intraperitoneal glucose tolerance test; IPITT, intraperitoneal insulin tolerance test; IRN, isorhynchophylline; ns, no significant.
Then we detected the effects of IRN on the inflammation of serum and placental tissues in GDM mice. In GDM mice treated with IRN, placental inflammation was identified through the presence of increased levels of inflammatory cytokines TNF-α, IL-6, and IL-1β. Analysis using ELISA assays revealed a decrease in the levels of these cytokines in both the serum and placental tissue of the IRN-treated groups when compared with the untreated GDM group (Figures 2a,b). The decrease in inflammatory markers suggests that IRN exhibits potent anti-inflammatory properties when used in GDM cases.
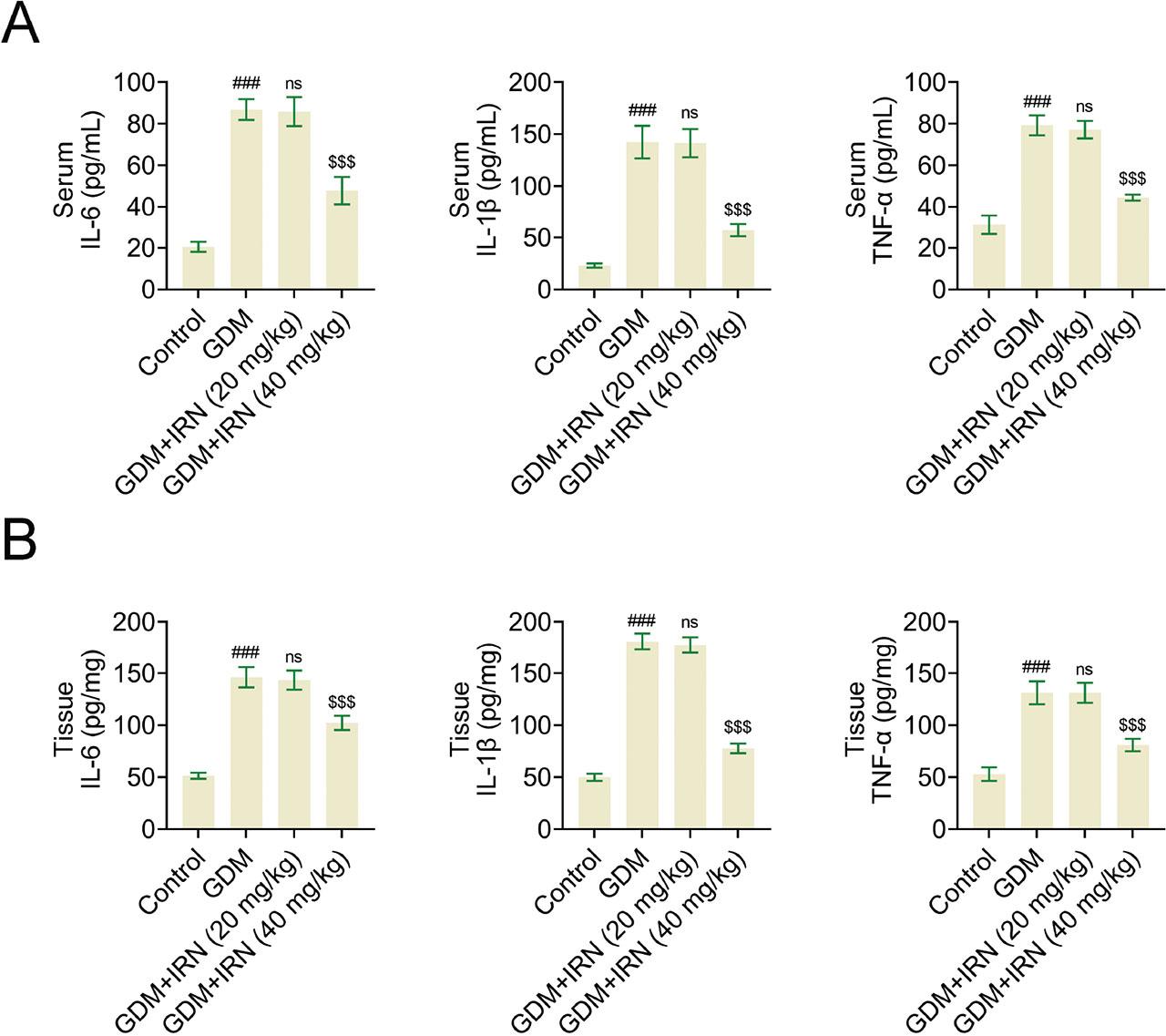
Effect of IRN on the inflammation of serum and placenta tissues of GDM mice. (A) Bar graphs of ELISA assays showing serum levels of inflammatory cytokines IL-6, IL-1β, and TNF-α in control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). (B) Bar graphs of ELISA assays showing placenta tissue levels of inflammatory cytokines IL-6, IL-1β, and TNF-α in control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). Data are presented as mean ± SD. *p < 0.001, GDM vs. control, **p < 0.001, GDM + IRN vs. GDM. ELISA, enzyme-linked immunosorbent assay; GDM, gestational diabetes mellitus; IL, interleukin; IRN, isorhynchophylline; ns, no significant; TNF, tumor necrosis factor.
In line with its anti-inflammatory effects, we next clarify the effects of IRN on the oxidative stress in the placenta. Oxidative stress markers, including MDA, SOD, GPx, and GSH, were measured in placental tissues. We noticed IRN treatment significantly reduced MDA levels and increased the activities of GPx, SOD, and GSH in GDM mice (Figure 3). This implies that IRN efficiently decreases oxidative stress in the placenta, thereby playing a vital role in enhancing placental functionality and promoting fetal growth.

Effect of IRN on the oxidative stress of tissues of GDM mice. The corresponding kits showed the levels of MDA, SOD, GPx, and GSH in placenta tissues from control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). Data are presented as mean ± SD. *p < 0.001, GDM vs. control, **p < 0.001, GDM + IRN vs. GDM. GDM, gestational diabetes mellitus; GPx, glutathione peroxidase; GSH, glutathione; IRN, isorhynchophylline; MDA, malondialdehyde; ns, no significant; SOD, superoxide dismutase.
The advantages of IRN therapy also positively impacted fetal growth. An increase in litter size was observed in the groups receiving IRN treatment in contrast to the untreated GDM group (Figure 4a), suggesting improved reproductive results with IRN therapy. In addition, the birth weight of offspring from IRN-treated GDM mice was higher compared with the untreated GDM group, indicating improved fetal development, although it remained lower than that of the healthy control group (Figure 4b). This implies that IRN therapy has a beneficial effect on the development of the fetus. To sum up, IRN enhances the quantity and weight of the offspring in GDM mice.

Effect of IRN on the litter size and offspring weight. (A) Litter size in control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). (B) Offspring weight in control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). Data are presented as mean ± SD. *p < 0.001, GDM vs. control, **p < 0.001, GDM + IRN vs. GDM. GDM, gestational diabetes mellitus; IRN, isorhynchophylline; ns, no significant.
Finally, the possible mechanism underlying IRN improving GDM in mice was investigated. The activation of the NLRP3 inflammasome pathway, a key regulator of inflammation, was detected in IRN-treated GDM mice. Interestingly, immunoblot analysis showed the reduced expression of NLRP3 (Figure 5a), caspase-1, IL-1β, and IL-18 (Figure 5b) in placental tissues of IRN-treated mice (Figure 5). This suggests that the anti-inflammatory properties of IRN may act by blocking the NLRP3 inflammasome pathway, thereby leading to enhanced metabolic and inflammatory results in mice with GDM.
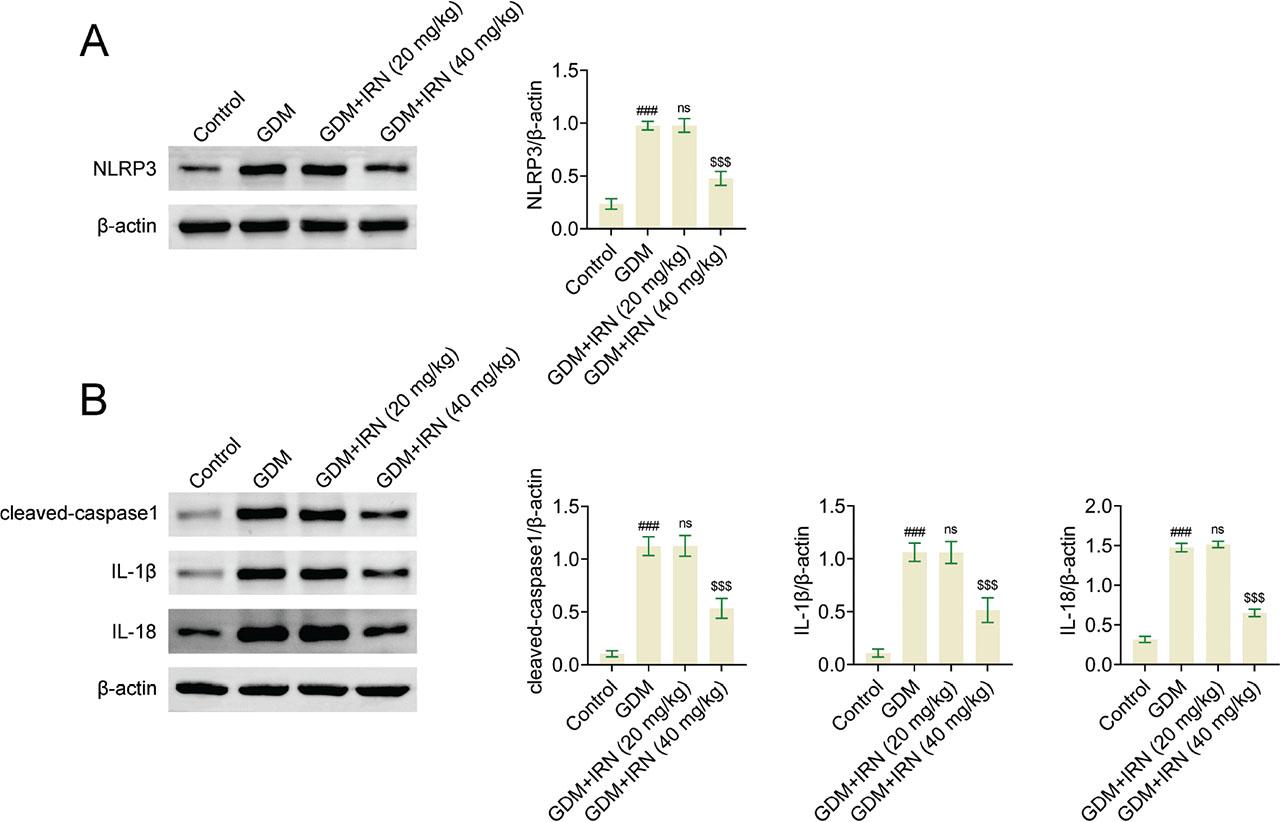
Effect of IRN on the NF-κB/NLRP3 pathway in GDM mice. (A) Immunoblot showed the expression of NLRP3 in placenta tissues from control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). (B) Immunoblot showed the expression of cleaved caspase-1, IL-1β, and IL-18 in placenta tissues from control, GDM, and IRN-treated GDM mice (20 mg/kg and 40 mg/kg). Data are presented as mean ± SD. *p < 0.001, GDM vs. control, **p < 0.001, GDM + IRN vs. GDM. GDM, gestational diabetes mellitus; IL, interleukin; IRN, isorhynchophylline; NF-κB/NLRP3, nuclear factor-κB/NOD-like receptor protein 3; ns, no significant.
In this study, we observed that GDM mice without IRN treatment (control group) exhibited significantly higher blood glucose levels and insulin intolerance compared with IRN-treated GDM mice (treatment groups). Research conducted in the past has shown that insulin resistance is a key characteristic of GDM, which arises from the body's ineffective utilization of insulin throughout pregnancy. The use of IRN has been found to enhance glucose metabolism and insulin sensitivity in GDM mice, indicating the capability of IRN to alleviate such metabolic irregularities. This effect may be due to IRN's ability to modulate inflammatory pathways and reduce oxidative stress, as inflammation and oxidative stress are known contributors to insulin resistance.
This study provides the first evidence of IRN's efficacy in improving GDM-related complications in vivo. By demonstrating its ability to modulate placental inflammation and oxidative stress, we establish a new therapeutic application for IRN in managing pregnancy-associated metabolic disorders. In contrast to traditional treatments for GDM, IRN not only lowers blood sugar levels but also helps to reduce inflammation and oxidative stress. This dual action targets the core issues of metabolic and placental dysfunctions that are key to GDM pathology.
While this study demonstrates that IRN reduces inflammation and oxidative stress in GDM mice, the precise molecular mechanisms remain to be fully elucidated. Based on our discoveries, it is indicated that the positive impacts of IRN may be achieved through the regulation of the NF-κB/NLRP3 pathway. Nonetheless, additional research is essential to validate the direct connections and subsequent outcomes in this signaling pathway.
Furthermore, we found that GDM mice had elevated blood lipid levels and reduced birth weights of offspring, which are consistent with the dyslipidemia and fetal growth restriction commonly associated with GDM. Additionally, the birth weight of offspring was higher in the IRN-treated groups compared with the untreated GDM group, indicating that IRN also positively impacts fetal development.
IRN is recognized for its ability to reduce inflammation, combat oxidative stress, and protect neurons (Zhao et al. 2021; Zheng et al. 2021; Li et al. 2022; Wang et al. 2023c). Numerous disease models have confirmed its capacity to regulate inflammatory reactions and oxidative stress (Li et al. 2022; Wu et al. 2023). In this study, IRN treatment reduced placental inflammation and oxidative stress in GDM mice, evidenced by decreased levels of TNF-α, IL-6, and IL-1β, as well as lower MDA levels and increased SOD and GSH activities. The findings indicate that the therapeutic benefits of IRN in managing GDM involve its ability to control inflammation and oxidative stress, both of which play vital roles in the development of GDM. By diminishing placental inflammation and oxidative stress levels, IRN has the potential to enhance placental functionality and promote optimal fetal growth, effectively tackling a significant complication associated with GDM.
Our findings also demonstrate that IRN inhibits the activation of the NLRP3 inflammasome pathway in GDM mice. The activation of the NLRP3 inflammasome plays a critical role in the regulation of inflammation and has been associated with the pathogenesis of insulin resistance and metabolic disorders (Yang et al. 2023b; Yangzhong et al. 2024). By inhibiting the NLRP3 inflammasome, IRN reduces the production of pro-inflammatory cytokines such as IL-1β and IL-18, thereby attenuating inflammatory responses and improving metabolic outcomes in GDM mice. This is in line with earlier studies indicating that addressing the NLRP3 inflammasome may improve metabolic irregularities linked to GDM. The suppression of the NLRP3 inflammasome by IRN may serve as a crucial mechanism by which it delivers its advantageous outcomes, offering a fresh therapeutic strategy for handling GDM.
The NF-κB/NLRP3 pathway plays a crucial role in regulating inflammation and oxidative stress, both of which are implicated in the pathogenesis of GDM (Liu et al. 2022). This study found that IRN exerts its beneficial effects on GDM by modulating this pathway. NF-κB, a transcription factor, regulates the activation of different inflammatory genes, whereas NLRP3 plays a vital role in the inflammasome complex responsible for the processing and release of pro-inflammatory cytokines (Wang et al. 2024; Zhou et al. 2024). IRN can improve insulin sensitivity and metabolic health in GDM mice by inhibiting the activation of NF-κB and NLRP3, thereby reducing the production of inflammatory cytokines and oxidative damage. This is consistent with previous research emphasizing the critical role of the NF-κB/NLRP3 pathway in regulating inflammation and oxidative stress in metabolic disorders.
Despite these promising results, this study has several limitations. The precise molecular mechanisms by which IRN modulates the NF-κB/NLRP3 pathway remain to be fully elucidated. Moreover, this research was carried out using a mouse model, and it is imperative to conduct additional studies to ascertain the relevance of these discoveries to humans. Subsequent research should also investigate the enduring impacts of IRN treatment on the health of both mothers and fetuses, along with its possible interactions with other medications. Understanding the detailed mechanisms and conducting clinical trials will be essential for translating these findings into clinical practice.
This study demonstrates that IRN significantly improves metabolic and inflammatory parameters in GDM through inhibition of the NF-κB/NLRP3 signaling pathway. Although these findings provide strong evidence of IRN's therapeutic potential, the precise mechanism by which it inhibits NLRP3 activation remains unclear. The chemical structure of IRN suggests that it may act as a Michael acceptor. This suggests that IRN may potentially establish covalent linkages with essential cysteine residues in the NLRP3 protein, thereby directly impeding its activation. Similar mechanisms have been observed with other covalent inhibitors targeting the NLRP3 inflammasome. Additionally, the presence of an ester group in IRN suggests its potential role as a prodrug, where in vivo enzymatic hydrolysis could lead to the release of an active metabolite that elicits biological responses. Although we did not perform in vitro experiments to confirm these hypotheses in this study, existing evidence indicates that IRN reduces inflammation and oxidative stress by modulating the NF-κB/NLRP3 pathway. Subsequent research will center on verifying the potential immediate inhibitory impacts of IRN on activating the NLRP3 inflammasome through sophisticated biochemical tests, like speck formation assays. Moreover, identifying the dynamic metabolite of IRN and the particular receptors it targets will offer more insights into its molecular process.
Further investigations are needed to establish if IRN has the ability to forestall the initiation of GDM by focusing on initial metabolic and inflammatory routes. Exploring its prophylactic potential could expand its application as both a preventive and therapeutic agent for pregnancy-related metabolic disorders.
In conclusion, this research shows that IRN effectively enhances metabolic and inflammatory indicators in GDM by blocking the NF-κB/NLRP3 signaling pathway. These findings highlight the therapeutic potential of IRN for managing GDM and improving maternal and fetal outcomes.