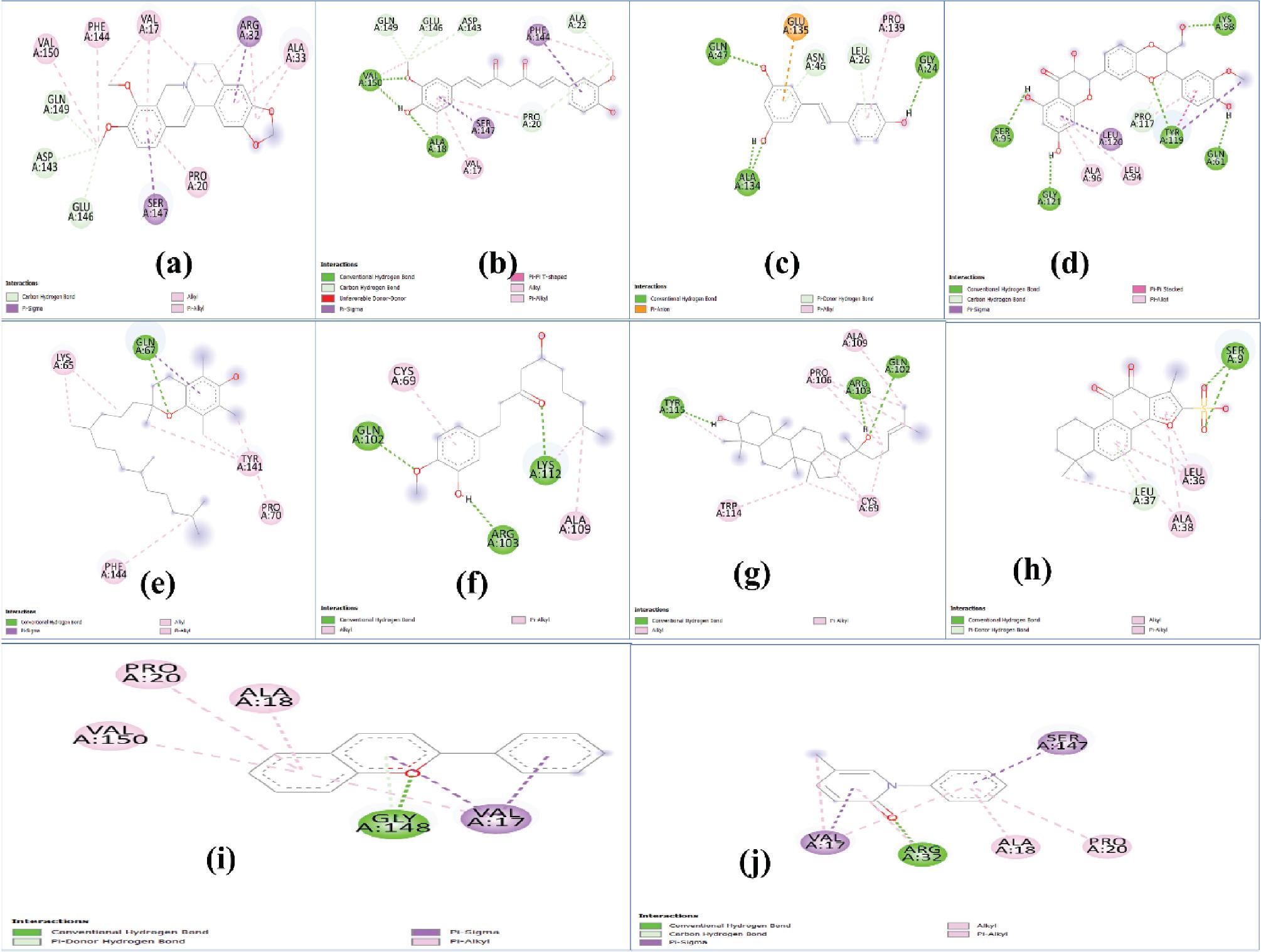
Figure 1.
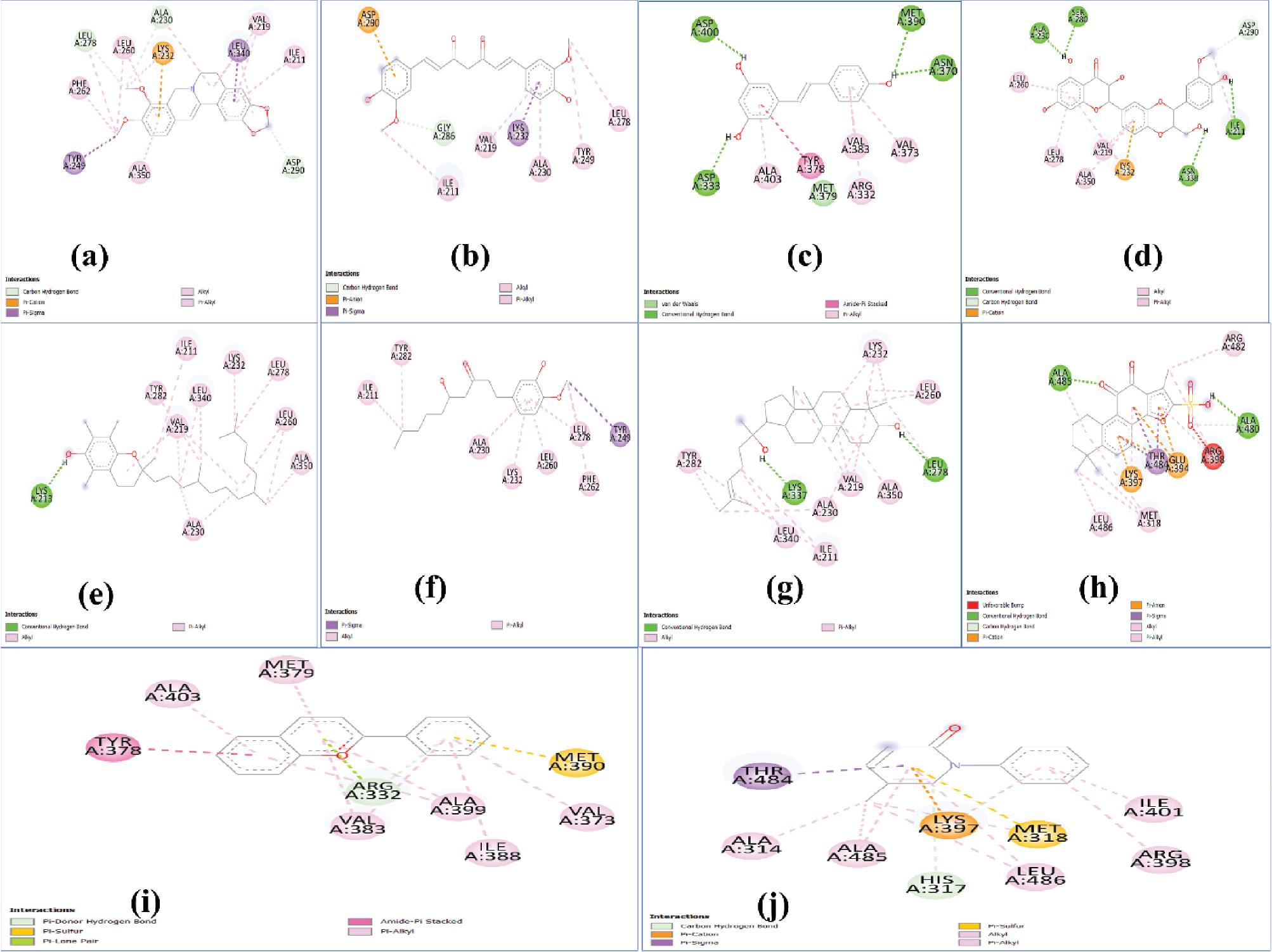
Figure 2.
The results of in silico docking study for different ligands with TNF-α
| Protein | Ligand | No. of hydrogen bonds | Interaction energy (kcal/mol) | Inhibition coefficient (μm) | Prominent bonds |
|---|---|---|---|---|---|
| TNF-α | Berberine | 0 | –7.11 | 6.11 | Pi sigma, pi alkyl |
| Curcumin | 3 | –7.17 | 5.54 | Hydrogen bond, Pi sigma, Pi T shaped | |
| Resveratrol | 4 | –5.88 | 49.02 | Hydrogen bond, Pi anion | |
| Silymarin | 5 | –7.21 | 5.17 | Hydrogen bond, Pi sigma, Pi stacked | |
| Tocopherol | 1 | –5.98 | 41.16 | Hydrogen bond, Pi sigma, pi alkyl | |
| Ginger | 3 | –3.91 | 0.011 | Hydrogen bond, pi alkyl | |
| Ginseng | 3 | –8.50 | 0.590 | Hydrogen bond, pi alkyl | |
| Danshen | 2 | –7.21 | 5.22 | Hydrogen bond, pi alkyl | |
| Anthocyanin | 1 | –7.41 | 3.72 | Hydrogen bond, Pi donor hydrogen bond, pi sigma, pi alkyl | |
| Pirfenidone | 1 | –6.33 | 22.81 | Hydrogen bond, pi sigma, pi alkyl |
The different etiologies of chronic liver disease in the context of fibrogenesis
| Pathogenesis | Study implications | |
|---|---|---|
| Viral hepatitis (Hepatitis B and C) | Viral hepatitis is a major cause of chronic liver inflammation and fibrosis. The hepatitis viruses directly infect hepatocytes, leading to immune-mediated liver damage, chronic inflammation, and subsequent fibrogenesis. TGF-β is known to play a crucial role in the fibrogenic response to viral infection by promoting the activation of HSCs and ECM deposition. | In the context of viral hepatitis, the inhibition of TGF-β by the herbal compounds identified in our study (e.g., danshen and ginseng) could be particularly beneficial in reducing fibrosis progression. TNF-α inhibition might also help mitigate the chronic inflammatory response triggered by viral infection, thus potentially slowing down the fibrogenic process [35–37]. |
| ALD | Chronic alcohol consumption leads to liver injury through oxidative stress, lipid peroxidation, and the production of pro-inflammatory cytokines, including TNF-α. Alcoholinduced activation of HSCs and the resulting ECM deposition are key contributors to fibrosis in ALD. The role of TGF-β in promoting fibrogenesis is also prominent in ALD. | The compounds that target TNF-α, such as ginseng, may help reduce inflammation and hepatocyte apoptosis associated with ALD [38]. Additionally, targeting TGF-β could be effective in mitigating the progression of fibrosis in ALD by reducing HSC activation and ECM accumulation [39]. |
| NAFLD and NASH | NAFLD and NASH are increasingly recognized as leading causes of liver fibrosis. The pathogenesis involves insulin resistance, lipid accumulation in hepatocytes, oxidative stress, and chronic inflammation. TGF-β plays a pivotal role in driving fibrosis in NASH by inducing the transformation of HSCs into myofibroblasts. TNF-α also contributes to inflammation and hepatocyte injury in NAFLD/NASH. | The findings suggest that the herbal compounds with strong TGF-β inhibitory effects, such as danshen, may be particularly effective in treating fibrosis in NAFLD/NASH [40]. Additionally, TNF-α inhibition by compounds like silymarin could help alleviate inflammation and slow down the progression to fibrosis [41]. |
| AIH | AIH is characterized by immune-mediated attack on hepatocytes, leading to chronic inflammation and fibrosis. The persistent activation of immune cells and the production of cytokines, including TNF-α, are central to the pathogenesis of AIH. TGF-β is also involved in the fibrotic response in AIH. | Herbal compounds that inhibit TNF-α and TGF-β may offer therapeutic benefits in AIH by reducing immune-mediated inflammation and subsequent fibrosis. The dual inhibition of these pathways could potentially slow the progression of fibrosis and improve liver function in patients with AIH [42, 43]. |
The results of in silico docking study for different ligands with TGF-β
| Protein | Ligand | No. of hydrogen bonds | Interaction energy (kcal/mol) | Inhibition coefficient (μm) | Prominent bonds |
|---|---|---|---|---|---|
| TGF-β | Berberine | 0 | –8.21 | 0.953 | Pi cation, pi sigma, pi alkyl |
| Curcumin | 0 | –6.06 | 36.41 | Pi cation, pi sigma, pi alkyl | |
| Resveratrol | 4 | –8.66 | 0.447 | Hydrogen bond, Amide pi stacked | |
| Silymarin | 4 | –8.09 | 1.18 | Hydrogen bond, Pi cation | |
| Tocopherol | 1 | –8.41 | 0.689 | Hydrogen bond, pi alkyl | |
| Ginger | 0 | –5.46 | 99.52 | Pi sigma, pi alkyl | |
| Ginseng | 2 | –10.83 | 0.011 | Hydrogen bond, pi alkyl | |
| Danshen | 2 | –11.58 | 0.003 | Hydrogen bond, Pi cation, pi anion, pi sigma, pi alkyl | |
| Anthocyanin | 0 | –8.73 | 0.396 | Pi donor hydrogen bond, Pi-sulfur, Pi lone pair, amide pi stacked | |
| Pirfenidone | 0 | –7.03 | 7.08 | Pi cation, pi sigma, pi sulfur, pi alkyl |