Aquatic ecosystems are regulated by dynamic interactions across three primary interfaces: the water column, suspended particulate matter (SPM), and bottom sediments. The SPM acts as a key agent in the transformation of substances, especially heavy metals (HMs). Moreover, the mobility and sorption properties of SPM require detailed study and evaluation (Li et al., 2022). The SPM comprises both organic and inorganic matter suspended within the water column and is quantitatively expressed through total suspended solids (TSS), typically measured in mg·L−1. All natural water bodies contain SPM to varying degrees, with their concentrations and characteristics being vital determinants of ecological balance (Xia et al., 2022). Anthropogenic activities have notably increased SPM concentrations, thereby altering key physical, chemical, and biological parameters of freshwater systems. The environmental impact of SPM is influenced by multiple factors: its concentration, exposure duration, and physicochemical properties, including particle size and chemical composition (Qasim, 2021). The particle size of SPM directly influences its suspension time and depth distribution, as well as its metal-binding capacity. Due to their colloidal nature and high surface area, smaller particles demonstrate higher sorptive potential, particularly for dissolved HMs. Consequently, SPM significantly contributes to the redistribution of metal pollutants in riverine ecosystems.
The impact of SPM on watercourse living organisms is, as a rule, greater at higher concentrations. Furthermore, the impact of living organisms’ exposure to concentrations of SPM is subject to variation according to period, duration of exposure, and the condition of the living organisms themselves. The chemical composition of SPM is also a critical factor because it controls its toxicity. The distribution of SPM particles by size is a critical factor in determining the duration of SPM suspension, as well as its distribution within the depth of the water reservoir and its sorption affinity (Fig. 1). SPM particles of a smaller size will be suspended for a greater period than particles of a larger size. SPM size has been demonstrated to exert a significant influence on sorption affinity. SPM particles, due to their colloidal nature, possess a high sorption capacity due to their small size. In the context of river water systems, SPM exhibits a high capacity for the sorption of dissolved HMs, attributable to its active nature and substantial surface area (Gantayat et al., 2023).
The distribution of heavy metals and the general scheme of this study
Source: compiled by the authors using AI graphical tools.
The distribution of HMs in river water systems can be affected by different control parameters, including pH, salinity, turbidity, and TSS. The use of correlation analysis facilitates the identification and prediction of the behavior of HMs (Kuznietsov & Biedunkova, 2025). The calculation of the distribution coefficient (Kd) facilitates the analysis of the distribution balance of HMs between the SPM and the liquid phase. The study by Kucuksezgin et al. (2007) demonstrates that variations in the Kd of the HMs between the SPM phase and the liquid phase are caused by water chemistry and TSS concentrations. Specifically, Kaiser (1989) also discovered that phytoplankton blooms enhance the amount of HMs in the SPM. The distribution of Cd, Cu, and Cr showed significant contamination in SPM, while Zn content showed a low degree of contamination. The transfer of free and bound forms of HMs to the SPM phase is associated with the formation of hydroxides that are insoluble in water, especially for Mn and Fe (Zhang et al., 2018). Nazeer et al. (2014) found that suspended sediment samples were more contaminated with Ni, Pb, and Cd compared to bottom sediment samples. In the study by Zeng et al. (2019), the occurrence of heavy metals had the following order of decreasing concentrations in SPM: Mn > Zn > V > Cr > As > Cu > Ni > Pb > Cd. According to the results of another study, the following order of enrichment of SPM with heavy metals was observed: Zn > Pb > Cr > Cd > Cu > Ni. At the same time, the distribution coefficient values of metals between solid particles and dissolved fractions had the following order: Pb > Cr > Zn > Cu > Cd > Ni (Zheng et al., 2013). Distinct distribution values obtained for disparate water bodies thus necessitate separate studies, as each possesses unique dynamics and hydrochemical parameters.
Among the various pollutants, HMs adsorbed by sediments are of particular interest due to their mobility and toxicity within the water ecosystem (Pourabadehei & Mulligan, 2016; Singovszka & Balintova, 2020). The deposition process and content of HMs in reservoir sediments have been extensively studied, but the distribution of HMs in explosives has been considered much less frequently. The scientific problem lies in the lack of quantitatively verified relationships between TSS/SPM particle size distribution, Kd partition coefficient, and the phase content of HMs for conditions of medium-sized rivers of the temperate zone; the practical problem lies in the lack of local thresholds and reference parameters (seasonal Kd by particle classes, event-based turbidity triggers), without which it is not easy to model HM mobility and plan effective water protection measures. SPM is an essential carrier of HMs in a water body, and the evaluation of HM contaminations in SPM is critical as a preliminary step towards effective management of surface water and a further base for water resource sustainability (Zeng et al., 2020; Idan et al., 2021; Wang et al., 2025). However, this is not a common occurrence in developing countries. The purpose of this research endeavor was to undertake a comprehensive examination of the TSS concentrations and SPM particles size distribution, with a view to identifying the distribution of HMs (Cd, Pb, Cr, Zn, Cu, Mn, Fe) between SPM and water in the Styr River (Fig. 1). The primary objectives are as follows: first, to investigate the dynamics of TSS concentration; second, to investigate the distribution of SPM particles by size; third, to investigate the relationship between TSS and HM concentrations; fourth, to investigate the dynamics of HM content in water and SPM; and fifth, to estimate the distribution of HMs in dissolved (water) and particulate (SPM) phases.
Thus, our study aims to fill these gaps for the Styr River through consistent measurement of TSS, SPM size composition, HM phase content, and the calculation of local Kd, increasing the applied value of monitoring and management decisions.
The Styr River water samples were collected monthly during the cold (January–March, October–December) and warm (April–September) periods, during 2024. The Styr River is in north-western Ukraine with a length of 483 km, a drainage basin of 13,130 km2, and an annual mean flow rate of 69 m3·s−1 (Stelmakh, 2024). Samples were taken at a point 183 km from the mouth of the Styr River (51°21ʹ53.2″ N, 25°51ʹ01.3″ E). The territory within this riverbed section belongs to the Polissya zone of Ukraine. The administrative location is the city of Varash, Rivne region, at the water intake site of the Rivne Nuclear Power Plant. Up to this point, the river is affected by agricultural, domestic, and industrial wastewater.
Figure 2 presents an overview of the study’s workflow. An amount of 1 L of water was filtered through blue strip filter paper to determine TSS using standard gravimetric procedures. The retained SPM on the filter was subjected to acid digestion using a CEM MARS 6 (microwave digestion system). Specifically, 50 mg of SPM was treated with 1.0 mL HF and 0.5 mL HNO3 in pre-treated Teflon vessels, heated sequentially to 140°C and 185°C for 4 hours, and followed by dissolution in 2.5 mL of 40% HNO3 at 135°C for another 4 hours. The digested solution was diluted to 50 mL with 2% HNO3. The HM concentrations in the leachate of water samples and after acid mineralization were analyzed using ICP-OES with an iICAP 7400 Duo spectrometer. Detailed information on the measurement method is available at EN ISO 11885 (International Standard Organization [ISO], 2019). The SPM characteristics of the particle size distribution with the determination of the median floc size (D50) were performed using a laser particle counter HIAC/ROYCO 8000A, according to the KND 211.1.4.039 recommendations (Ministry of Environmental Protection and Nuclear Safety of Ukraine [Mіndovkіllya], 1995).
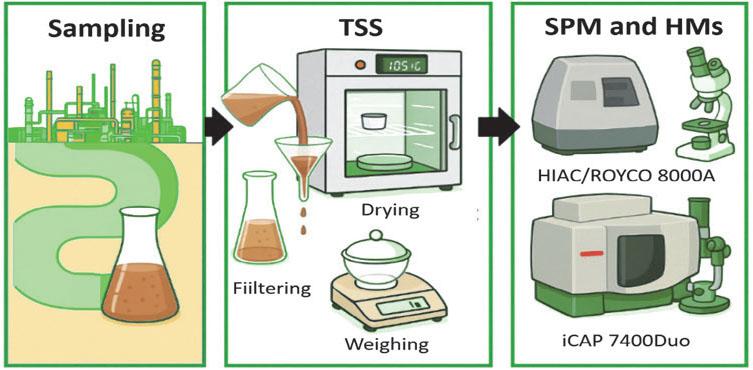
General scheme of sampling, sample preparation, and measurements
Source: compiled by the authors using AI graphical tools.
The distribution coefficient of heavy metals between the dissolved phase (water) and particulate (SPM) phases was calculated using the following formula (Cao et al., 2023):
A higher Kd value (beyond 3) indicates a higher affinity of the metals for SPM or absorption, while a value below 3 means a higher affinity of the HMs for the dissolved phase.
The statistical processing of the research results involved determining the arithmetic mean (M) and the standard deviation (SD). In addition, the correlation between HM concentrations was determined using the Pearson correlation coefficient (r). The JASP software package Version 0.14.3 was used for the statistical processing.
The distribution of TSS levels suggests a statistically significant increase in TSS levels during the warm period (Table 1). Thus, the M ±SD TSS concentration was 12.5 ±2.7 mg·L−1 in warm and cold periods, and the M ±SD TSS concentration was 18.3 ±3.0 mg·L−1. The observed seasonal variations can be attributed to multiple environmental and hydrodynamic factors (Walch et al., 2022). In the warm period, intensified biological activity, sediment resuspension due to enhanced flow turbulence, and increased precipitation-driven runoff contribute to elevated TSS levels. Conversely, during the cold period, reduced biological productivity and lower flow velocities may lead to greater sediment deposition, thereby reducing TSS concentrations.
Concentrations of total suspended solids during cold and warm periods
| Period | M [mg·L−1] | SD [mg·L−1] | min–max [mg·L−1] |
|---|---|---|---|
| Cold | 16.2 | 7.3 | 5–30 |
| Warm | 21.7 | 7.1 | 10–35 |
Source: own work.
In the cold period, the distribution of SPM floc sizes was such that D50 equals 11.4 μm, indicating the predominance of small particles (Table 2). In contrast, during the warm period, D50 increases significantly to 24.8 μm, indicating the presence of larger particles and aggregation. Warmer conditions favor microbial activity, organic matter formation, and the strengthening of polymeric bonds, which contribute to the formation of larger flocs. In addition, the increased turbulence in the warm period promotes frequent particle collisions, leading to greater aggregation. Conversely, in colder conditions, reduced biological exudation and lower turbulence limit floc growth, resulting in the formation of smaller, more dispersed particles. Increases in TSS concentrations and floc size during the warmer months indicate a greater propensity for sediment transport and potential impacts on water clarity, nutrient content, and water resources.
Morphological differences in the suspended particulate matter flocs in cold and warm periods
| Feature | Cold period | Warm period |
|---|---|---|
| Visual appearance | small, dispersed, fine flocs | larger, more aggregated flocs |
| Dominant floc size range (min–max) [µm] | 8–15 | 20–30 |
| Floc size D50 [µm] | 11.4 | 24.8 |
| Structural compactness | more discrete, less aggregated | more compact and cohesive structures |
Source: own work.
The Pearson correlation analysis of HM concentrations in both the dissolved phase (water) and the SPM phase provides critical insights into the mechanisms governing metal transport, partitioning, and seasonal variability in aquatic systems. In the dissolved phase (Fig. 3), TSS exhibit weak or negligible correlations with most HMs, indicating that, in their dissolved state, these elements remain largely independent of the SPM. Therefore, solubility affects their distribution more than adsorption on SPM. However, strong positive correlations (r > 0.7) are observed among Fe, Mn, and Cr, suggesting a common geochemical origin or co-dissolution processes. Conversely, Cd shows weak correlations with most metals, which may indicate differences in its geochemical behavior, potentially driven by distinct sources, redox sensitivity, or competitive interactions with other ions.
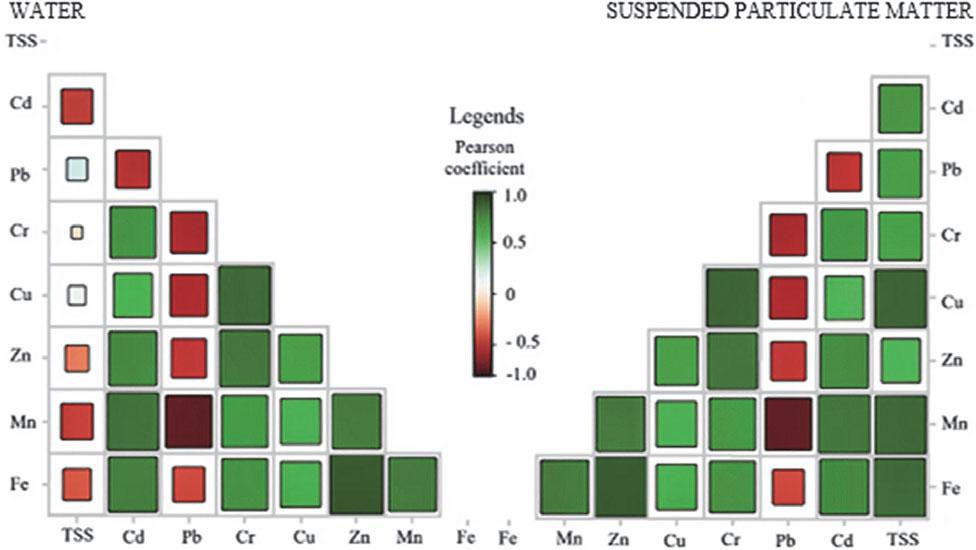
The Pearson correlation matrix of heavy metal concentrations in dissolved and particulate phases
Source: own work.
Negative correlations, such as those observed between Fe and Zn and Mn, further suggest that different metals follow distinct pathways in the dissolved phase, likely due to variations in their affinities for complexation with organic ligands or adsorption onto mineral surfaces. In contrast, in the SPM-associated phase (Fig. 3), HM correlation with TSS is substantially stronger, particularly for Fe, Mn, and Zn. This finding suggests that higher TSS concentrations enhance the adsorption of these metals onto suspended particulates, thereby increasing their transport in the particulate-bound phase. Strong correlations, particularly among Fe, Mn, Cr, and Pb (r > 0.7), indicate that these elements are primarily associated with iron and manganese oxides or organic-bound fractions within SPM. Meanwhile, Cd demonstrates only weak associations with other metals in SPM, further supporting the notion that it remains more mobile in its dissolved form compared to other HMs. During the warm period, increased TSS concentrations, as previously demonstrated (Table 1), enhance HM adsorption onto particulates, leading to stronger correlations between TSS and HMs in the SPM phase (Fig. 3).
The seasonal distribution of HMs between dissolved and particulate phases, as detailed in Table 3, offers valuable insight into their environmental dynamics. The proportion of individual НМs within each phase and across periods demonstrates substantial variability, indicative of their distinct physicochemical properties, binding tendencies, and environmental reactivity under differing seasonal conditions. Fe and Mn consistently constitute the largest fraction of total metals in both the dissolved and particulate phases, a pattern that aligns with their prevalent occurrence as oxides and hydroxides in natural aquatic systems. However, distinct seasonal patterns emerge: during the cold period, elevated proportions of Cu, Zn, Pb, Cr, and Cd are found in the dissolved phase, whereas the warm period is marked by increased concentrations of Fe and Mn. Among the HMs analyzed (Table 4), the sequence of decreasing phase affinity follows: Fe > Mn > Cd > Pb > Cu > Zn > Cr.
Content of heavy metals in dissolved and particulate phases during cold and warm periods
| Phase/Period | HM content [%] | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Mn | Cu | Zn | Pb | Cr | Cd | |
| Water/cold period | 47.4 | 25.6 | 4.7 | 3.1 | 6.9 | 7.8 | 4.5 |
| Water/warm period | 50.2 | 34.7 | 3.2 | 1.3 | 4.0 | 5.1 | 1.5 |
| Suspended particulate matter/cold period | 85.1 | 8.3 | 1.8 | 1.0 | 2.0 | 1.1 | 0.7 |
| Suspended particulate matter/warm period | 89.4 | 6.0 | 1.4 | 0.9 | 1.2 | 0.6 | 0.5 |
Source: own work.
Distribution coefficient (Kd) values for heavy metals (HMs) and phase affinity interpretation
| HM | Kd | Phase affinity | ||
|---|---|---|---|---|
| min | M | max | ||
| Cd | 15.40 | 33.20 | 77.60 | high absorption onto the solid phase |
| Pb | 2.20 | 7.80 | 18.20 | high absorption onto the solid phase |
| Cr | 0.02 | 0.11 | 0.31 | high dissolution into the liquid phase |
| Cu | 0.30 | 1.21 | 2.05 | intermediate absorption |
| Zn | 0.12 | 0.63 | 0.71 | tendency toward dissolution |
| Mn | 53.10 | 104.30 | 502.10 | high absorption onto the solid phase |
| Fe | 203.10 | 455.60 | 707.20 | very strong absorption onto the solid phase |
Source: own work.
Intermediate Kd values observed for Cu and Cd suggest partial affinity toward SPM, although a substantial portion remains dissolved. This behavior is likely regulated by interactions with organic ligands and redox-sensitive solubility. Zn, exhibiting the lowest Kd values, demonstrates a clear preference for the aqueous phase, indicative of its higher solubility and weaker adsorption capacity (Antoniadis et al., 2007). These findings are consistent with previous studies indicating that Zn contamination is typically found in dissolved form rather than associated with particulates. In summary, the behavior of HMs in aquatic environments is largely governed by their chemical characteristics and environmental context (Liu et al., 2024). Fe and Mn serve as dominant sorbents and carriers within SPM, while HMs like Zn and Cr tend to remain dissolved due to lower sorption potential.
The observed seasonal fluctuations of the SPM and related HMs in the Styr River reflect the dynamics between hydrological, thermal, and geochemical processes. During the warmer months, elevated temperatures and increased biological activity promote the aggregation of finer particles into larger flocs (median size increasing from 11.4 μm to 24.8 μm), enhancing the capacity of SPM to bind with particle-reactive metals like Fe, Mn, Pb, and Cr (Fig. 4).
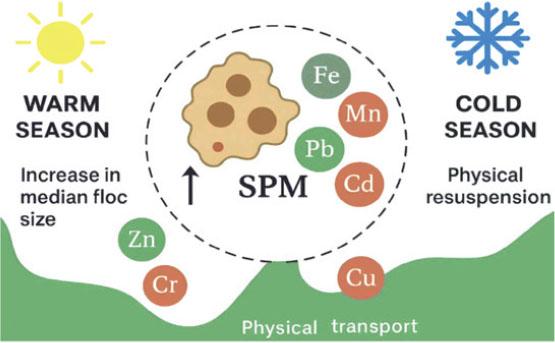
Complex pollution characteristics associated with suspended particulate matter and heavy metals
Source: compiled by the authors using AI graphical tools.
This trend aligns with findings by Liu et al. (2023), who reported that over 70% of HMs in the Yellow River were transported via SPM, with higher concentrations during rainy periods due to runoff-driven sediment loads. Our results also support the particulate-phase dominance of Fe and Mn (Kd > 3), while Zn and Cu remained primarily in the dissolved phase, suggesting differential geochemical behavior and binding affinities, consistent with reports by Xia et al. (2023) on the Yarlung Tsangpo River, where Cu and Zn exhibited weaker particulate associations.
Moreover, the stronger Pearson correlations between TSS and certain metals suggest that physical transport mechanisms (e.g., fluvial resuspension) rather than chemical transformations play a predominant role in metal mobility during high-flow periods. The Kd differentiation observed in our study corroborates the seasonal fluctuation models of HMs transport by Kuznietsov and Biedunkova (2023), emphasizing how SPM not only governs binding but also its seasonal flux across aquatic systems (Biedunkova & Kuznietsov, 2025). Neglecting SPM-associated fractions may significantly underestimate HM load, particularly during warm or flood-prone periods. Given the significant variation in HM behavior across physicochemical gradients and periods, long-term monitoring strategies should encompass both dissolved and particulate fractions for effective riverine metal pollution control.
The evaluation of HM distribution is essential for effective water quality management. Observations reveal significant temporal and spatial variations in HM concentrations, influenced by hydrometeorological events, fluvial discharge, and anthropogenic inputs (Fig. 5).
Assessment of the distribution of heavy metals (HM) in dissolved and particulate phases
Source: compiled by the authors using AI graphical tools.
According to Gong et al. (2016), TSS levels tend to rise during precipitation events, particularly when accompanied by moderate to high rainfall intensities and peaks, leading to increased TSS loads in runoff. In urban settings, dynamic TSS behavior is commonly observed in highway runoff, influenced by the proportion of fine (below 45 μm) and coarse particles, depending on prior dry periods (da Pereira Silva et al., 2024). Similarly, in tidal rivers, TSS exhibits high variability, which can be modeled to simulate sediment transport and estimate metal loads entering marine systems (Yao et al., 2021). Finer SPM particles possess a higher adsorption capacity for HMs, enhancing their role as carriers in aquatic environments (Li et al., 2023).
There is a well-established correlation between TSS and particulate--bound HMs such as Zn, Pb, and Cu in stormwater runoff. In river systems like the Yellow River, fine clay particles were found to significantly regulate HM concentrations, particularly during colder seasons. Comparable behavior was documented in the Xiaolangdi Reservoir, where resuspended sediments function as HM sinks, reducing downstream bioavailability (Dong et al., 2018). However, under certain geochemical conditions, SPM may also act as a source of HMs due to interactions with carbonate fractions or variations in organic matter.
Environmental factors such as salinity, sediment concentration, and dissolved organic matter significantly influence HM distribution between dissolved and particulate phases. In the Pearl River Estuary, HM concentrations were found to be lower upstream compared to downstream locations, particularly during warm periods, which was attributed to shifts in salinity and sedimentation rates (Du et al., 2019). TSS serve as an effective proxy for evaluating particle quality and size distributions in stormwater, assisting in the development of targeted mitigation strategies (Wang et al., 2018).
This study highlights the critical role of SPM for moving and separating HMs in water ecosystems. Seasonal variations significantly influenced both the concentration and morphology of SPM, with warmer periods promoting particle aggregation and enhancing the sorption capacity for particle-reactive HMs such as Fe, Mn, Pb, and Cd. In contrast, metals like Zn, Cu, and Cr remained predominantly in the dissolved phase, reflecting their lower affinity for SPM. The Kd and r analyses confirmed that metal mobility and phase distribution are strongly governed by environmental conditions, particularly temperature, turbulence, and biological activity. Ultimately, incorporating SPM-based assessments can improve the accuracy of ecological risk evaluations and inform effective strategies for surface water pollution management. The obtained parameters can be directly used for monitoring setups (including SPM and Kd in the list of indicators), determining TSS thresholds as sampling triggers, optimizing treatment plant and water treatment regimes, forming local standards and risk maps for the Styr sub-basin. The practical result will be an increase in the accuracy of environmental risk assessment, early warning of episodes of increased HM mobility, and support for management decisions in water use.
