Nuclear power is developing rapidly due to its high energy potential and environmental benefits (Danish, 2021; Zhan et al., 2021). Although nuclear power offers significant economic and social benefits, it produces radioactive wastes (RAWs) with varying levels of hazard and radioactivity. In terms of protection of the environment, the safe disposal of these RAWs has become an increasingly serious problem in a way that hinders the further development of the nuclear industry (Liu et al., 2024). Three radionuclides – 137Cs (T1/2 = 30.2 y), 90Sr (T1/2 = 28.2 y) and 79Se (T1/2 = 3.26×105 y) – occur in the produced RAWs with significant risk characteristics, such as relatively high radioactivity levels, long half-lives and significant radiation energies (Abass et al., 2022; Durmus & Erenturk, 2023). The potential release of radionuclides from RAWs poses serious risks to the environment and human health. Consequently, it becomes necessary to ensure their proper and safe treatment and storage (Liu et al., 2024).
Currently, more than 60% of electricity in the Slovak Republic is generated by nuclear power and this share is steadily increasing due to the gradual closure of thermal power plants as significant producers of carbon dioxide and other greenhouse gases. However, nuclear power plants (NPPs) are responsible for producing huge amounts of RAWs and spent nuclear fuel. The production of solid RAWs was on an upward trend from 2005 to 2020 at the Mochovce NPP, rising from 14.6 t in 2005 to 16.5 t in 2020. In contrast, at the Jaslovské Bohunice NPP, the production of solid RAWs was down by 49.9% over the same period. In the case of liquid RAWs, a significant decrease in their production was observed in the period 2005–2020, with a decrease from 127.5 m3 in 2005 to 11.7 m3 in 2020 in Mochovce and from 48.0 m3 to 15.1 m3 in Jaslovské Bohunice (these data do not include the amount of RAWs produced in the framework of the decommissioning of the A1 and V1 power plants by JAVYS, a.s.) (Enviroportál, 2023).
In Slovakia, JAVYS, a.s. is practically the only company responsible for the implementation of the treatment and storage of the above mentioned RAWs, as well as institutional RAWs (JAVYS, 2024).
In order to ensure the safe disposal of RAWs, a number of variables need to be considered that lead to the most appropriate disposal method for a particular type of RAW. There are different pre-treatment processes, RAW treatments and final storage systems. As mentioned above, in addition to the conventional methods (e.g. adsorption, chemical precipitation, extraction, membrane separation, etc.), attention is also being given to alternative remediation technologies, such as biosorption and phytoremediation for the separation of radionuclides using biological systems (e.g. microorganisms or plants) as a first step in the treatment of RAWs. In the next step, the aim is to achieve a reduction in the volume of RAWs (e.g. contaminated biomass), e.g. by incineration, which results in contaminated ash. The final step in the treatment of RAWs is to convert the contaminated material into a safe form with respect to its final storage. Solidification is used to convert RAWs into a solid and long-term stable form. Apart from cementation, as the longest used method of final RAW treatment, technology based on the production of geopolymer matrices is proving to be a suitable method of RAW solidification.
The concept and notion of geopolymers was first introduced in 1972 by French scientist Joseph Davidovits (Davidovits, 2011). Geopolymers belong to a group of mineral binders related to natural zeolites. The term geopolymers refers to aluminosilicate inorganic polymers with an amorphous three-dimensional network structure of a silica-oxygen tetrahedron and an aluminum-oxygen tetrahedron connected via an oxygen bridge (Hu et al., 2020). They are prepared using alkaline activation without the presence of a lime component and thus belong to the group of alkali-activated materials. The material, which is formed by the geopolymerization of an active aluminosilicate precursor and an alkali activator, is distinguished by excellent mechanical properties, high durability, resistance to acid and high temperatures, and low cost and carbon dioxide emissions (Amran et al., 2020). Due to these excellent properties, geopolymers are widely used in various manufacturing fields, such as building blocks (Singh et al., 2020), coatings (Lv et al., 2019), adhesives (Kürklü & Görhan, 2019) and refractories (Gao et al., 2020), and for the immobilization of hazardous wastes and materials (Fu et al., 2020) and emergency repairs (Bhutta et al., 2019).
In 2024, an interesting review paper was published by Liu et al. (2024), which addresses the solidification performance and mechanism of typical radioactive nuclear waste by geopolymers and geopolymer ceramics. It highlights the fact that for the last two years, papers dealing with the solidification and adsorption of cesium by cement, metakaolin- and fly ash-based geopolymers have been published in scientific databases. The use of metakaolin-based geopolymers in the solidification of cesium has been studied in works by Tian et al. (2022), Mukiza et al. (2023), and Tan et al. (2024). In recent years, inexpensive precursors for the preparation of geopolymers for the solidification of hazardous wastes have also been explored, of which fly ash has received significant attention (Wang et al., 2018; Pu et al., 2024). In this context, several works have shown that fly ash-based geopolymers may be suitably applicable in the solidification and immobilization of radiocesium 137Cs (Tian et al., 2019; Jain et al., 2022a; Jain et al., 2022b). Li et al. (2013) described the solidification of Cs+ by fly ash-based geopolymer as an encapsulation matrix and discovered that geopolymers could better meet the safe disposal requirements of radioactive waste compared to cement.
Based on these facts, it seems to be an interesting idea to use fly ashes obtained after the incineration of radioactive wastes containing 137Cs radiocesium or to apply fly ashes as adsorbents of 137Cs occurring in liquid radioactive wastes and subsequently solidify them using geopolymers. The aim of this work was to evaluate the possibility of solidifying fly ash contaminated with radiocesium 137Cs into geopolymer matrices. Within the individual experimental steps, the work focused on the preparation of fly ash originating from the Vojany thermal power plant (TPP) (Slovak Republic) and artificially contaminated with the radionuclide 137Cs, on the design and verification of the recipe for solidifying fly ash into a geopolymer matrix (commercially available under the name Geocem, produced by GEOFIX, a.s., Slovak Republic), and on evaluating the qualitative parameters of the final product in terms of its long-term storage in the RAW repository.
A three-component geopolymer mixture commercially available under the name Geocem, produced by GEOFIX, s.r.o. (Trnava, Slovak Republic), was used in the preparation of the geopolymer matrices. This mixture consists of three basic components, namely Geosil A consisting of sodium polysilicate, sodium hydroxide and potassium hydroxide; Geosil B containing calcium oxide, silicon oxide, aluminum oxide and magnesium oxide; and Geosil C including calcium oxide, silicon oxide and aluminum oxide.
The fly ash as waste studied came from the Vojany TPP (Slovak Republic). The sample was obtained at the end of 2020, as fly ash K5 (annual sample collection). The physico-chemical analysis of this fly ash was carried out by Eurofins Environment Testing Slovakia, s.r.o. (Slovak Republic), which included the determination of selected metals and semi-metals (Sb, As, Ba, Be, Bi, B, Sn, Al, Mg, Cr, Cd, Cu, Ni, Pb, Hg, Se, Tl, Te, V, Ca, Fe, and Zn) using different analytical methods (HG-AAS, ICP-OES, F-AAS, AAS-AMA, or UV-Vis), oxides (K2O, P2O5, Al2O3, MgO, SiO2, MnO, Na2O, TiO2, CaO, and Fe2O3), specific weight, solids content, loss of organic matter by annealing at 550°C, total sulfur, pyrite sulfur, sulfate sulfur, combustible matter at 830°C, loss by drying, dry matter at 105°C, bulk density, and determination of total chlorine. In addition, grain size testing, according to ČSN EN ISO 17892-4 (Česká agentura pro standardizaci [ČSN], 2017), and at the Výskumný ústav pre hnedé uhlie (Research Institute for Brown Coal) in Most (VUHU, a.s., Czech Republic), determination of the percentage of water, and the pH of the fly ash reaction according to STN ISO 10390 (Slovenski inštitut za standardizacijo [STN], 2005) were carried out.
Before the artificial contamination of the fly ash with the radioisotope 137Cs, the possible level of this radionuclide was analyzed by scintillation gamma-spectrometry in a sample of original fly ash (1 kg) placed in a Marinelli-type beaker. An accurately weighed quantity of fly ash was transferred into an Erlenmeyer flask containing a CsCl solution in deionized water spiked with 137CsCl of known activity, chemical concentration, and pH value. The pH of the solution was adjusted by the addition of 1 mol⋅dm−3 NaOH solution to a final value of pH 5.5. The exposure was carried out under stirring on a rotary shaker (150 min−1) at 25°C and the risk of evaporation of the water from the solution was prevented by covering the neck of the flask with parafilm. After 48 h of exposure, fly ash with bound 137Cs activity was collected on filter paper. Subsequently, an aliquot of the solution was removed and filtered through a syringe filter (13 mm diameter; 0.45 μm permeability) to remove any residual fly ash, and the residual 137Cs activity in the solution was determined using a scintillation gamma-spectrometer. The captured fly ash was allowed to dry in a laboratory oven at 40°C for several days to a constant weight.
Plastic samplers with a circular base with a diameter of 35 mm and a height of 50 mm were designed and fabricated to prepare the cylindrical geopolymer waste forms. Before loading the geopolymer matrix, these plastic samplers were covered with parafilm on the base to prevent the unsolidified geopolymer matrix from leaking out of the samplers.
The preparation of the geopolymer mass containing different percentages by weight of fly ash (5%, 10%, 20%, and 40%) artificially contaminated with 137Cs followed the proposed recipes shown in Table 1. From each variant, four samples were prepared in the form of cylinders of the geopolymer waste forms. The samples were allowed to cure for 45 days under laboratory conditions (25°C).
Proposed recipes for preparation of geopolymer waste forms containing different percentages by weight of fly ash (5%, 10%, 20%, and 40%) artificially contaminated with 137Cs
| Geopolymer waste forms with proportion of fly ash [wt.%] | Fly ash [g; d.w.] | Geosil A [g; d.w.] | Geosil B [g; d.w.] | Geosil C [g; d.w.] | Technical water [g] | Final product [g] | NaOH [g; d.w.] |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.00 | 42.00 | 24.00 | 123.00 | 111.00 | 300.00 | 15 |
| 5.00 | 15.00 | 38.67 | 22.10 | 113.23 | 111.00 | 300.00 | 15 |
| 10.00 | 30.00 | 35.33 | 20.19 | 103.48 | 111.00 | 300.00 | 15 |
| 20.00 | 60.00 | 28.67 | 16.38 | 83.95 | 111.00 | 300.00 | 15 |
| 40.00 | 120.00 | 15.33 | 8.76 | 44.91 | 111.00 | 300.00 | 15 |
Source: own work.
In the first step, the initial 137Cs activity bounds in a given formed geopolymer waste form was determined based on the known specific activity of 137Cs (Bq⋅g−1; d.w.) in the used, artificially contaminated fly ash sample, the percentage by weight of this fly ash in the final product, and the total dry weight of the formed geopolymer waste forms.
The leachability test was performed according to ANSI/ANS-16.1 (American National Standard [ANSI], 1986). The test itself was carried out in sealed plastic beakers with a total volume of 1 dm3, to which was added an exact volume of deionized water (0.05 μS⋅cm−1) calculated as ten times the surface area of the sample of the geopolymer waste form. The geopolymer waste form sample was then immersed in the deionized water and exposed under laboratory conditions at 25°C. After 2 h, the sample was removed from the water and immersed in a new plastic beaker of fresh deionized water of identical volume as above. The water sample obtained after exposure was analyzed for pH, conductivity, and 137Cs activity released from the geopolymer waste form using Marinelli-type beakers and scintillation gamma-spectrometry. Similar procedures were subsequently followed for exposure times of 4 h, 7 h, 24 h, 48 h, 72 h, 96 h, and 120 h.
As the fly ash was artificially contaminated with radiocesium 137Cs, its long half-life of T1/2 = 30.2 y allows us to ignore its radioactive decay during the testing in terms of the activity determined in the leachates. Under such conditions, the effective diffusivity can be calculated using the following mathematical relationship (Eq. 1):
The leachability index Li is then calculated using the following equation (Eq. 3):
The compressive strength test of the formed geopolymer waste form was carried out using a prototype of a piece of compressive strength measurement equipment (GEOFIX, s.r.o.). It was a manual hydraulic press with an integrated manometer and a maximum pressure of 20 t. The compressive strength measurement was preceded by the pressing of the geopolymer waste form samples into perfectly flat surfaces. The prepared sample was inserted between the contact surfaces of the hydraulic press and the pressure on the sample was increased by stepwise pumping. The pressure at which sample destruction occurred was then read on the manometer.
A scintillation gamma-spectrometer with a 3″ diameter NaI(Tl) well-type crystal 76BP76/3 (Envinet, 2024) coupled with the evaluation program ScintiVision-32 (Ortec, USA) was used to determine the initial and residual 137Cs activity in the solutions from the experiments aimed at artificial contamination of fly ash with radionuclide 137Cs, and to determine the bound 137Cs activity directly in the fly ash. The detector was placed in a cylindrical lead shield with a height of 250 mm and a wall thickness of 35 mm of Pb to minimize the background effect. For the leachate samples obtained from the leachability test, the 137Cs activity was measured on an identical scintillation detector, but with the difference that the leachate samples with a volume of 600‒800 cm3 were analyzed in Marinelli-type beakers (type 1040G; max. volume 1,000 cm3) and on a detector placed in a lead shield designed for high-resolution gamma-spectrometry (Envinet, Czech Republic; height 400 mm, wall thickness 100 mm Pb). The scintillation gamma-spectrometers were calibrated in terms of γ-photon energies and measurement efficiencies using the above evaluation program and the built library of analyzed radioisotopes: 109Cd (Eγ = 88.04 keV), 137Cs (Eγ = 661.66 keV), and 60Co (Eγ = 1,173.24 keV), using standard solutions of the given radioisotopes. The calibration considered the geometry of the measurement, either in the form of vials inserted into the detector well or in the form of Marinelli-type beakers placed on the detector. The duration of the sample analysis was 600 s, which ensured a relative measurement uncertainty of up to 2%.
The obtained data were statistically evaluated and processed into graphical dependencies using Origin Pro 2016 (OriginLab Corporation, 2016) and MS Excel 2016 (Microsoft Corporation, 2016).
In the first step, we characterized in detail the fly ash sample studied. Based on the results obtained, we can say that the studied fly ash sample did not contain heavy metals (Cu, Zn, Hg, Cr, Cd, or Pb) in concentrations that would be abnormal or unnatural for the given sample type. We also found that the fly ash sample studied contained naturally high concentrations of the elements Al, Mg, and Ca, which also form an important component within the geopolymers. Al was present in the fly ash sample at a concentration of 107 g⋅kg−1 (10.7%), Ca at 101 g⋅kg−1 (10.1%), and Mg at 10.5 gkg−1 (1.05%).
Other important chemical components of geopolymers in terms of their final structure and polymerization include oxides, in particular aluminum oxide, magnesium oxide, silicon oxide, and calcium oxide. For this reason, analyses were also carried out to determine the abundance of oxides in the studied fly ash sample. We found that the fly ash sample contained silicon oxide at a percentage by weight of 39.5%, aluminum oxide at 20.2%, calcium oxide at 14.1% and magnesium oxide at a percentage of 1.74% (Table 2). It should be noted at this point that the mechanism of the effect of some oxides, such as CaO and SO3, on the mechanical properties and dimensional stability of fly ash-based geopolymers is not clear. Chen et al. (2022) observed that the mechanical properties of fly ash-based geopolymers initially increase and then decrease with the increasing CaO and SO3 content.
Proportion of oxides in fly ash sample originating from Vojany TPP (Slovak Republic)
| Oxide | Weight proportion [%; d.w.] | Measurement uncertainty [%] | Analytical method |
|---|---|---|---|
| K2O | 2.01 | 10 | F-AAS |
| P2O2 | 0.53 | 15 | ICP-OES |
| Al2O3 | 20.2 | 15 | ICP-OES |
| MgO | 1.74 | 15 | ICP-OES |
| SiO2 | 39.5 | not determined | gravimetry |
| MnO | 0.12 | not determined | ICP-OES |
| Na2O | 0.66 | 10 | F-AAS |
| TiO2 | 0.81 | not determined | ICP-OES |
| CaO | 14.1 | 15 | ICP-OES |
| Fe2O3 | 6.78 | 20 | ICP-OES |
ICP-OES – inductively coupled plasma optical emission spectrometry; F-AAS – flame atomic absorption spectrometry.
Source: own work.
In the final analyses, we focused on the abundance of basic elements such as sulfur in its various chemical forms, and total chlorine, as well as on the solids content, loss of organic matter by annealing at 550°C, combustible matter at 830°C, loss by drying, dry matter determined at 105°C, bulk density, and specific weight. These analyses were supplemented by the determination of the pH reaction of the fly ash in suspension with water (weight ratio of fly ash to deionized water equals 1 : 5). We found that the fly ash showed a strongly basic character with a pH of 12.2.
Based on these facts, it can be assumed that the studied fly ash represents a suitable material that chemically complements the basic components of the geopolymer mixture, which implies that the evaluated fly ash can be applied to this mixture in significant weight proportions, representing tens of percent. This assumption is also the basis of the extensive review work by Li et al. (2022), which deals with the preparation, properties, and application possibilities of fly ash-based geopolymers.
For the artificial contamination of a fly ash sample with 137Cs, we found that with the increasing CsCl concentration in deionized water, the percentage of 137Cs bound activity by the studied fly ash sample decreased significantly. The conducted experiments also showed that the amount of initial 137Cs activity in the deionized water had a significant effect on the 137Cs specific binding values obtained in Bq⋅g−1 d.w. The specific 137Cs activity bound in the individual fly ash samples ranged from 420 Bq⋅g−1 (d.w.) to 969 Bq⋅g−1 (d.w.). The fly ash samples thus artificially contaminated with radiocesium 137Cs were subsequently applied for the preparation of geopolymer waste forms containing different percentages by weight of fly ash (5%, 10%, 20%, and 40%), whose physico-chemical and geometric characteristics are presented in Table 3.
Physico-chemical and geometric characteristics of prepared geopolymer waste forms
| Proportion of fly ash [%] | Average weight of samples [g] | Average height of samples [cm] | Average sample volume [cm3] | Average surface area of samples [cm2] | Initial 137Cs activity of samples [Bq] |
|---|---|---|---|---|---|
| 5.00 | 58.0 ±0.3 | 4.25 ±0.03 | 40.9 ±0.3 | 66.0 ±0.4 | 2 137 |
| 10.00 | 52.3 ±0.5 | 4.80 ±0.06 | 46.2 ±0.6 | 72.1 ±0.6 | 6 475 |
| 20.00 | 45.1 ±0.3 | 4.64 ±0.02 | 44.6 ±0.2 | 70.2 ±0.3 | 6 543 |
| 40.00 | 35.7 ±3.8 | 5.31 ±0.51 | 51.1 ±4.9 | 77.6 ±5.6 | 5 830 |
Source: own work.
Many authors point out that several factors will play an important role in the preparation of fly ash-based geopolymers for the successful and highly efficient solidification or immobilization of Cs, as well as the microstructure of the formed geopolymers. Jain et al. (2022a; 2022b) found that the critical process parameters are the curing time and curing temperature, followed by the interaction between the curing temperature and the Cs dosage. They also explained (Jain et al., 2022a) that the Cs dosage affected the resulting phase compositions and pore structure of fly ash-based geopolymers, eventually influencing the Cs immobilization in these matrices. In our case, we worked with the radioisotope 137Cs and simulated radioactive wastes originating from NPP operations, which are characterized by very low Cs concentrations but not negligible radiochemical characteristics. In addition, Tian et al. (2019) showed that a high Si/Al ratio could contribute to a more compact of geopolymer, as confirmed by the decrease in the pore volume with the increasing Si/Al ratio. In contrast, the geopolymer with a low Si/Al ratio had a higher Cs+ immobilization efficiency than the geopolymer with a high Si/Al ratio due to the high adsorption density.
The leachability test performed according to the ANSI/ANS-16.1 standard is implemented in the operations of the National Radioactive Waste Repository (NRWR) in Mochovce (Slovak Republic) as an important parameter determining the suitability of the solidified RAWs for their storage.
While performing the leachability test and at the specified times of 2 h, 4 h, 7 h, 24 h, 48 h, 72 h, 96 h, and 120 h, the 137Cs activity in the whole volume of deionized water was determined, and the pH and conductivity of the water were measured. Figure 1 shows graphically the changes in the pH, conductivity, and 137Cs activity values in the deionized water released from the prepared geopolymer waste forms with different fly ash contents. As can be seen, there was a gradual decrease in the above measured values at short time periods, i.e. after 2 h, 4 h, and 7 h of exposure. A similar dependence was observed for measurements that proceeded at daily intervals, i.e., after one day, two days, three days, four days, and five days of exposure. In the case of conductivity, we found that the highest values of conductivity, in mS⋅cm−1, probably originating from the release of chemical components of the prepared geopolymer waste forms, were observed for the samples exhibiting the lowest fly ash proportions. The conductivity values ranged from approx. 1 mS⋅cm−1 to 5 mS⋅cm−1. This was not clearly demonstrated for the pH values of the water; the pH of the deionized water increased from an initial pH 5.5 to pH values of 10.6‒11.8 within the individual times of measurement. Regarding the 137Cs activity released into the water, the opposite phenomenon was observed, namely that the highest values of the measured activities were observed for the geopolymer waste form samples containing the highest applied content of fly ash (40 wt.%).
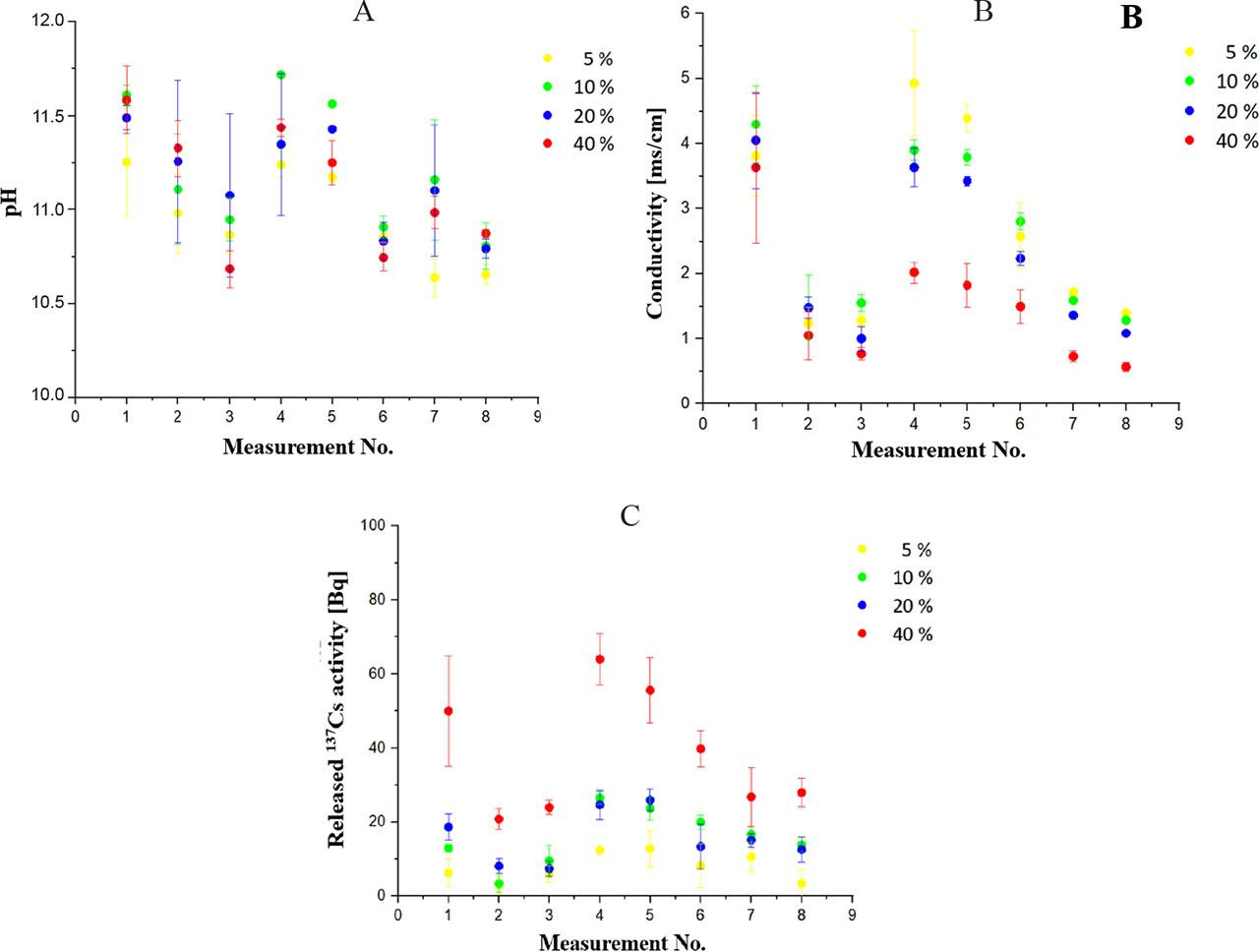
Changes in pH (A), conductivity (B), and 137Cs activity (C) values in deionized water released from prepared geopolymer waste forms with different fly ash contents (5 wt.%, 10 wt.%, 20 wt.%, and 40 wt.%). After each measurement, the deionized water was replaced with fresh water and the duration of exposure of the geopolymer waste form sample from the beginning of the leachability test was as follows: 1st measurement – after 2 h of exposure; 2nd measurement – after 4 h; 3rd measurement – after 7 h; 4th measurement – after 24 h; 5th measurement – after 48 h; 6th measurement – after 72 h; 7th measurement – after 96 h; 8th measurement – after 120 h of exposure. The values represent the arithmetic means and the error bars describe the standard deviation of three independent experiments
Source: own work.
We also evaluated the 137Cs leachability within individual samples and measurements in the context of the initial 137Cs activity determined in a given cylinder of the geopolymer waste forms through the applied fly ash contaminated with 137Cs. We found that the highest percentage releases of 137Cs activity within the individual measurements were observed for the geopolymer waste form samples containing fly ash at 40% by weight. At the same time, these results showed that we confirmed a slightly higher percentage release of 137Cs activity than 1% in only one case of exposure time.
Within this leachability test and in accordance with the ANSI/ANS-16.1 standard, parameters such as the effective diffusivity (D) and, in particular, the dimensionless leachability index (Li) were evaluated, based on which solidified RAWs are determined to be suitable or unsuitable for storage in RAW repositories. This standard defines that if the Li value is greater than 6, the solidified RAWs are suitable for storage in an RAW repository. Conversely, if the Li value is less than 6, the solidified product does not meet the criteria for storage in a repository, e.g. the NRWR in Mochovce. Based on the calculated Li values, we found that the prepared geopolymer waste form samples with 5%, 10%, and 20% fly ash content by weight showed Li values ranging from 6.34 to 6.54, and thus met this criterion. For the sample with 40% fly ash content, we obtained a value of Li equal to 5.58, from which we can conclude that this solidified RAWs did not pass the leachability test and therefore cannot be stored in a RAW repository.
Jain et al. (2022b) found that for prepared fly ash-based geopolymers and Cs immobilization, the leachability index Li ranged from 7.4 to 14.6 (determined according to the ANSI/ANS-16.1-1 standard), which significantly exceeded the minimum acceptable value of 6. They explained these very high Li values via the combination of the chemical (within the amorphous gel and zeolitic phase) and physical (adsorbed on surfaces and trapped within porosity) immobilization of Cs in fly ash-based geopolymers. They also emphasized that the remarkable improvement in the performance is due to the formation of the Cs-bearing zeolitic phase (pollucite) found in properly processed fly ash-based geopolymers.
As in the leachability test, the compressive strength test of solidified RAWs is considered a critical parameter determining their suitability or unsuitability for storage in RAW repositories planned for hundreds of years. In each country, the value of the pressure at which the solidified RAW is destroyed may vary according to the standards adopted for this purpose. In the conditions of the Slovak Republic, a minimum pressure on the wall of a solidified RAW of at least 5 MPa has been set for a given solidified RAW to be able to resist pressure. In Figure 2, we present the results of the material compressive strength tests for the individual prepared geopolymer waste form samples. As can be seen, the samples with fly ash contents ranging from 0 wt.% to 35 wt.% met the above limit. At the same time, we found that the value of the pressure at which the destruction of a given sample occurred decreased from a value of approx. 12 MPa determined for the sample without fly ash addition to a value of 8 MPa for the geopolymer waste form sample containing 35 wt.% of fly ash. As with the leachability test, the sample containing 40 wt.% fly ash proved to be unsuitable for storage in the RAW repository, with the destruction occurring at a pressure of only 4 MPa.
From the point of view of comparing our results concerning the effect of fly ash content in geopolymer waste forms on their compressive strength with the results reported in scientific databases, there are papers that show a different pattern of this dependence. For example, Carrillo-Beltran et al. (2021), when studying the effect of the percentage amount of fly ash originating from the combustion of olive tree biomass in prepared geopolymers (from 10 wt.% to 40 wt.%) on their compressive strength, found that this parameter increased with the increasing fly ash percentage up to 30 wt.% and then started to decrease slightly, reaching compressive strength values in the tens of MPa in all the cases analyzed. Also, Teixeira et al. (2022) confirmed in their work the synergistic effect of adding fly ash derived from biomass combustion on improving the characteristics of concrete containing fly ash derived from coal combustion.
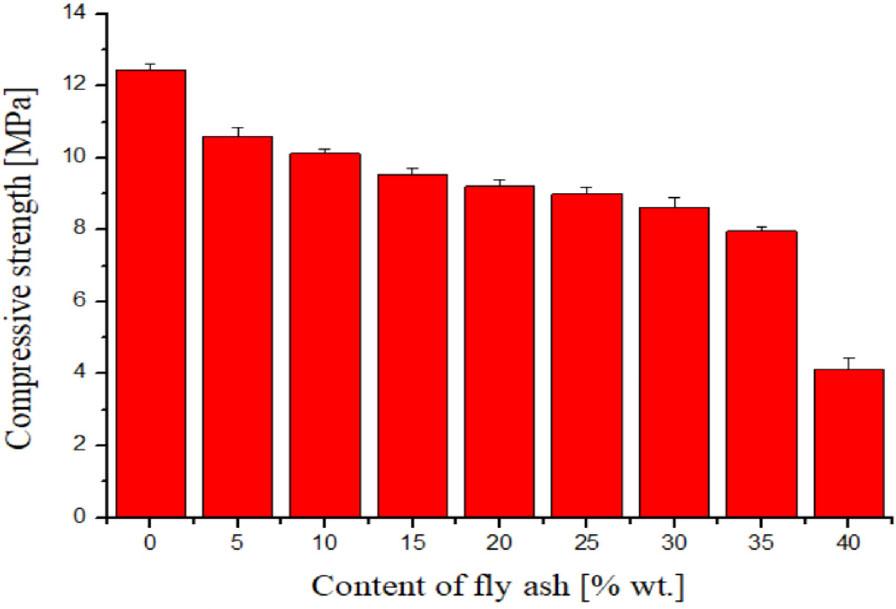
Compressive strength measured for prepared geopolymer waste forms with different fly ash contents. The values represent the arithmetic means and the error bars describe the standard deviation of three independent experiments
Source: own work.
This work investigated the possibility of solidifying fly ash contaminated with radiocesium 137Cs into geopolymer matrices (commercially available under the name Geocem, produced by GEOFIX, s.r.o., Slovak Republic). The fly ash sample originated from the Vojany TPP (Slovak Republic) and was artificially contaminated with the radionuclide 137Cs. The main aim of the study was to design and validate the recipe for solidifying fly ash into a geopolymer matrix such that the final product meets the qualitative leachability and compressive strength test parameters to allow its long-term storage in the NRWR in Mochovce.
In terms of physico-chemical characterization of the fly ash sample, it was found that the fly ash sample studied contained naturally high concentrations of the elements Si, Al, Mg, and Ca, especially in the form of their oxides, which also form an important component within geopolymers.
The fly ash was artificially contaminated with radiocesium 137Cs through its relatively short-term exposure (48 h) in a 137CsCl solution. These experiments showed that with the increasing CsCl concentration in deionized water, the percentage of 137Cs bound activity by the studied fly ash sample decreased significantly. Also, the initial 137Cs activity in the deionized water had a significant effect on the specific 137Cs activity bound in the individual fly ash samples subsequently used to prepare geopolymer matrices containing different percentages by weight of fly ash (5%, 10%, 20%, and 40%) ranging from 420 to 969 Bq⋅g−1 (d.w.).
The leachability test carried out according to the ANSI/ANS-16.1 standard for five days, which is also adopted in the NRWR in Mochovce, showed that the geopolymer waste forms with fly ash contents of 5%, 10%, and 20% met the required limits of the leachability index Li being more than 6. For the sample with a fly ash content of 40%, this index reached the value of Li less than 6, which indicates the unsuitability of this material for storage in the NRWR in Mochovce.
The compressive strength test confirmed that the geopolymer waste forms with a fly ash content of 40% did not meet the required 5 MPa limit for its disposal. Samples with fly ash contents ranging from 0 to 35 wt.% met the above limit. At the same time, it was found that the value of the pressure at which the destruction of a given sample occurred decreased from a value of approx. 12 MPa, determined for the sample without a fly ash addition, to a value of 8 MPa, measured for the geopolymer waste form sample containing 35 wt.% of fly ash.
Based on the obtained results, it can be concluded that the geopolymer mixture used is capable of absorbing significant amounts of fly ash as RAW representing up to 30% of the total mass of the geopolymer waste forms prepared, while also meeting the required limits determined by the leachability and compressive strength tests that have been adopted in the operation of the NRWR in Mochovce as a RAW repository.
