Climate change and drought are closely interrelated, as climate change significantly influences precipitation patterns, leading to drought in many regions of the Earth. Climate change refers to long-term shifts in average meteorological conditions, including temperature, precipitation, wind, and other atmospheric phenomena (Cook et al., 2018). The growing global demand for water, coupled with the impacts of climate change, is placing immense pressure on water resources (Oladosu et al., 2019). Abiotic factors, such as temperature, salinity, and drought, are key drivers affecting agricultural production. These factors are projected to increasingly contribute to soil degradation and reduced crop yields. Thus, it is crucial to develop innovative agricultural practices that enhance water use efficiency. Superabsorbent polymers (SAPs) are one potential solution in this regard.
Superabsorbent polymers (SAPs) are three-dimensional cross-linked hydrophilic polymers capable of absorbing and storing large quantities of water, which can be gradually released during periods of drought. Since the American Department of Agriculture made the first SAP available in the 1970s, SAPs offer promising applications in a variety of fields, including modern agriculture, materials for personal hygiene products, biomedicine, drug delivery systems, sewage treatment, construction, healthcare, electronics and so on for their excellent water-absorption and retention capabilities. Currently, SAPs are widely used in products, such as diapers, sanitary napkins, tampons, and nursing pads (Ahmed, 2015; Mignon et al., 2019; Prasad et al., 2023; Chavan et al., 2024; Wang et al. 2024a).
SAPs could be groundbreaking technologies for the adaptation of agriculture to climate change. When applied to cultivated soil, SAPs absorb and retain water from irrigation (both artificial and natural) and prevent rapid loss through drainage and evaporation. As the soil dries, the stored water is released from the hydrogel via diffusion, keeping the soil or substrate moist for extended periods. The application of hydrogels improves soil density, structure, and permeability, enhances water infiltration, and reduces evaporation rates, all while decreasing water runoff and soil erosion, ultimately leading to improved crop productivity (Nnadi & Brave, 2011; Frioni et al., 2024). Another advantage of SAPs is their ability to retain nutrients in the soil and serve as carriers for slow-release fertilizers. In addition to improving soil water-use efficiency, SAPs are also used for the controlled release of fertilizers. It has been reported that approx. 40–70% of the nitrogen (N) and 80–90% of the phosphorus (P) in conventional fertilizers cannot be absorbed by crops due to their high solubility and rapid diffusion into the surrounding environment (Hou et al., 2017).
SAPs can be classified based on various factors, including chemical composition, source, and application. SAPs are generally classified from the point of view of their origin as natural, synthetic, or semisynthetic. Natural SAPs, such as those derived from cellulose, starch, and chitosan, are easily biodegradable, but they have a lower water absorption capacity and must therefore be used in larger quantities. On the other hand, synthetic SAPs, such as polyacrylic acid and polyacrylamide, have a higher water absorption capacity and are more cost-effective, but their lower biodegradability may negatively impact the environment and plant growth (Chang et al., 2021; Chavan et al., 2024). Semisynthetic polymers, which combine the properties of both natural and synthetic polymers, are frequently used in applications like drug delivery. For instance, chitosan can be crosslinked with polyacrylic acid for use in medicinal delivery systems (Chang et al., 2021; Chavan et al., 2024).
In recent years, driven largely by increasing concerns about environmental sustainability, there has been growing interest in developing biodegradable SAPs for commercial agricultural applications. The development of biodegradable SAPs from renewable resources is gaining significant attention from scientists and researchers worldwide. In contrast to synthetic SAPs, biodegradable alternatives offer ecological benefits and contribute to environmental protection. Many companies that produce hydrogel products are now focusing on the development of environmentally friendly absorbent materials derived from renewable resources, rather than synthetic SAPs made from petroleum byproducts (Mali et al., 2018; Calcagnile et al., 2019; Godwin et al., 2019; Chavan et al., 2024). Cellulose and its derivatives present a sustainable alternative due to their renewable, biodegradable, and abundant characteristics, and show promise as a source for the production of eco-friendly SAPs (Dodangeh et al., 2024).
SAPs consist of three-dimensional crosslinked hydrophilic networks capable of absorbing and retaining vast amounts of water. This water absorption capacity is a key parameter used to describe SAPs, defined as the ratio of the weight of absorbed water [g] to the dry weight of the SAP [g]. While retaining their physical structure, three-dimensional hydrophilic networks of polymer chains can absorb, expand, and hold aqueous solutions up to 1,000-time their weight, while standard SAPs can retain a maximum of 10-times their weight (Chen et al., 2022; Prasad et al., 2024; Wang et al., 2024b). A typical SAP is defined as a highly hydrophilic material whose absorption mechanism is based on osmotic pressure, which is a direct result of its extreme hydrophilicity and cross-linked structure. SAPs draw water into their three-dimensional network through osmotic pressure, thanks to the compatibility between water and polymer chains. To achieve excellent absorption properties, SAPs must contain a sufficient number of strongly hydrophilic groups, which are generally highly polar or ionic (following neutralization by metal ions – M). Examples include –OH, –COOH/–COO-M, –CONH2, –SO3H/–SO3-M, and others (Dodangeh et al., 2024).
There are four primary mechanisms by which absorbent materials absorb water: (i) reversible changes in their crystal structure (e.g. silica gel and anhydrous inorganic salts); (ii) physical capture of water by capillary forces in macroporous structures (e.g. soft polyurethane sponges); (iii) a combination of capillary action and hydration of functional groups (e.g. tissue paper); (iv) a combination of the above with the natural dissolution of hydrophilic polymer chains, which is the primary mechanism of SAP formation (Matjašič et al., 2021).
Two types of cross-linking are commonly used in SAP production: bulk cross-linking and surface cross-linking. Bulk cross-linking, also known as core cross-linking, usually occurs during the polymerization stage of SAP production. For example, in the polymerization of acrylic acid, a dialkene cross-linking agent is used, and its molecules are incorporated directly into the main polymer chain during radical polymerization. This process results in SAPs with higher cross-linking densities, which provide greater structural stability and gel strength. However, this may reduce their water absorption due to the dense cross-linking, which limits the free space for water molecules. Once the pores allowing water entry into the core become blocked, further water penetration is prevented, leading to “gel blocking” (Kiatkamjornwong, 2007).
To mitigate this issue, surface cross-linking methods are now widely used. In this process, a surface coating helps maintain sufficient gel strength while allowing adequate water absorption into the core. Surface cross-linking typically occurs on a side group (e.g. carboxyl group) of a preformed base polymer (Dabhi et al., 2013).
The aim of this study was to assess the effect of soil nutrients on the stability of superabsorbent polymers (SAPs). SAPs derived from starch and mixtures of acrylic acid and acrylamide were tested. First, a physicochemical characterization of the SAPs was performed, which included optimization of the water absorption volume, determination of the maximum water absorption capacity, and analysis of the water binding kinetics. Then, we also investigated the effects of selected nutrients and metals on the water absorption capacity of SAPs.
A sample of superabsorbent polymer (SAP) was obtained from PEWAS, Ltd. (Bratislava, Slovakia) under the trade name AquaVantage AV200. The polymer is a complex mixture consisting of 60% (by weight) starch-based polymers and 40% (by weight) of a blend of acrylic acid and acrylamide (in a weight ratio of 10 : 1). It appeared as a fine white-cream colored powder with a grain size of 200 mesh. The sample was stored in a dry environment until the experiments were conducted. Before use, the SAP was dried in a hot air oven at 60°C for 24 h.
The water absorption capacity of the SAP was evaluated through experiments using two types of gravimetric methods: (i) the tea bag method for evaluating hydrogel formation, and (ii) the test tube method using a Microfil filtration system (Millipore, 2017) with membrane cellulose filters.
The objective of the first experiment was to determine the water absorption capacity of the SAP, i.e., the maximum volume of water that the polymer can absorb. A precisely weighed 100 mg sample of the SAP was placed into a pre-weighed empty tea bag. The tea bag containing the SAP was then placed at the bottom of a two-liter plastic container, and specific volumes of ultrapure water (30 mL, 40 mL, 50 mL, 75 mL, 100 mL, 200 mL, and 500 mL) were added. Three parallel samples were tested for each volume. After 2 h, the tea bag with the swollen hydrogel was removed from the water, allowed to drip for 10 s, and then weighed. The water absorption capacity of the polymer was calculated using the following equation (Eq. 1):
The second experiment focused on evaluating the swelling kinetics of the hydrogel. The procedure was identical to that of the water volume optimization experiment, but using 100 mL of ultrapure water as the optimized volume. Measurements were taken at time intervals of 5 min, 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, and 1,440 min, with three parallel samples for each time of exposure. The hydrogel swelling capacity was calculated using the same equation (Eq. 1).
As an alternative method for evaluating the swelling kinetics, the gravimetric method using a Microfil filter system and pre-weighed cellulose membrane filters (47 mm in diameter) was employed. A five-milligram sample of SAP was weighed into a test tube, followed by the addition of 10 mL of ultrapure water. The contents were mixed in a vortex mixer for 5 s. Samples were taken at the same time intervals as in the case of the tea bag method (5 min, 15 min, 30 min, 60 min, 120 min, 180 min, 240 min, and 1,440 min) with three parallel series. The water-hydrogel mixture was then separated into the filter system, excess water was removed using the filtration system, and the hydrogel was retained on the filter. The hydrogel swelling capacity was calculated using the same equation (Eq. 1).
To evaluate the stability of the hydrogel in the presence of Hoagland’s medium (Hoagland, 1920), test tube experiments were conducted using a gravimetric analysis. An exact amount of 5 mg of SAP was weighed into test tubes (two parallel series), and prepared solutions of Hoagland’s medium (10% and 100% of ionic strength) were added to the tubes. After the solutions were added, the samples were vortexed for uniform mixing. Following a two-hour swelling period, the samples containing the formed hydrogel were separated onto the filter system, and the solution was removed using a vacuum filtration system. The hydrogel retained on the filter was then weighed, and the hydrogel swelling capacity was calculated using Equation (1).
To assess the stability of the hydrogel in the presence of nutrients and metal ions at concentrations equimolar to the potassium content in the SAP, test tube experiments were conducted with a gravimetric analysis using the Microfil filtration system. Metal solutions of Na, K, Zn, Co, Cu, Cs, and Cd (in the form of chlorides) were prepared in 200 mL volumetric flasks, each with a concentration equimolar to the potassium content in the SAP. A precise amount of 5 mg of the SAP was weighed into test tubes (three parallel series), and 10 mL of each prepared metal solution was added. The samples were then vortexed. After a two-hour swelling period, the samples were filtered through the Microfil filter system, and the hydrogel was retained on the filter and weighed. The hydrogel swelling capacity was calculated according to Equation (1).
Determination of the potassium (K) in a mineralized SAP sample was performed using an ATS 200MKI flame photometer (Switzerland). A calibration curve and the equation of the calibration line for calculating the concentration of K in the SAP sample were obtained using standard K solutions in the concentration range of 5–100 mg·L−1. During the analysis of both the standard calibration solutions and the mineralized SAP sample, the solution was atomized using a nebulizer and combusted in a non-luminous propane-butane/air flame at temperatures between 1,600°C and 1,900°C. The concentration of K in the SAP sample was calculated based on the measured signal and by substituting the result into the equation derived from the calibration curve for determining K.
To determine the potassium content in the analyzed SAP, a gamma-spectrometric analysis was performed using a 76BP76/3scintillation gamma-spectrometer equipped with a NaI(Tl) well-type crystal and lead shielding for high-sensitivity and high-resolution gamma-spectrometry (Envinet, 2024). The analysis was carried out using the ScintiVision-32 evaluation program (Ortec, USA). Initially, a background measurement was conducted with a measurement time of 5,000 s. Following this, a standard containing a known quantity of potassium, specifically the radioisotope potassium-40 (40K) in the form of potassium chloride (KCl), was measured using Marinelli containers (1040G). The weight of the measured standard was 1,200 g. During the measurement, a peak with an energy of 1,460 keV was detected, which is characteristic of gamma-photons emitted during the decay of 40K. The measurement time for the standard was 5,000 s. Subsequently, a sample of the analyzed SAP, weighing 983 g, was measured under the same conditions. Based on the measured activity of 40K in the sample, the potassium content in the analyzed SAP was determined.
The data processing and evaluation were performed using the Microsoft Excel (Microsoft Corporation, 2016) and Origin Pro 2016 (Origin Lab Corporation, 2016) software.
The initial phase of the experiments focused on optimizing the conditions for hydrogel formation, specifically the volume of the absorbate and the kinetics of the hydrogel swelling process. Once the optimal conditions were determined, further experiments were conducted to evaluate hydrogel formation in different solutions, including Hoagland’s medium and in the presence of metal ions.
The first experiment was aimed at optimizing the volume of water to determine the water absorption capacity of the SAP, i.e., the maximum volume of water that the SAP can absorb. Various water volumes were tested to establish the optimal hydrogel swelling capacity of the polymer.
Based on Figure 1, the trend of the water absorption capacity of SAP increases linearly with the volume of added water up to 100 mL. Beyond this point, the water absorption capacity shows minimal change, even as the water volume increases. Consequently, 100 mL was chosen as the optimal volume for determining the water absorption capacity in subsequent experiments, as further increases did not significantly improve this water absorption. It can therefore be concluded that after 2 h of exposure of SAP to various volumes of water, the SAP was saturated with water molecules from a volume of 100 ml and thus the maximum water absorption capacity was achieved.
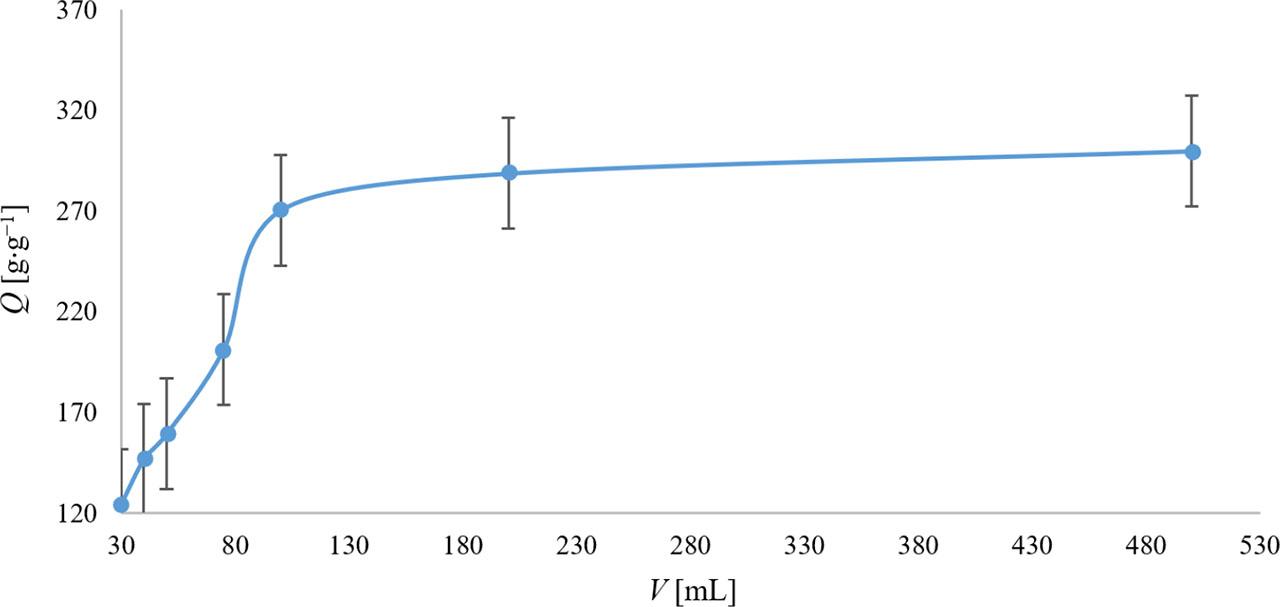
Dependence of SAP’s water absorption capacity (Q) on water volume (V). The values represent the arithmetic means, and the error bars describe the standard deviation of three independent experiments
Source: own work.
The results from the volume optimization experiment were further analyzed using the Origin 2016 program to determine the maximum water absorption capacity of the SAP with ultrapure water as the sorbate. The obtained data were fitted by Langmuir’s adsorption isotherm to provide a deeper understanding of the water absorption behavior.
In Figure 2, the maximum water absorption capacity (Qmax) of the SAP for ultrapure water, based on Langmuir’s adsorption isotherm, is shown to be 322 ±18 g⋅g−1 (parameter a in the graph). However, it is important to note that during the experiments using tea bags, a partial release of the hydrogel through the tea bag material was visually observed.
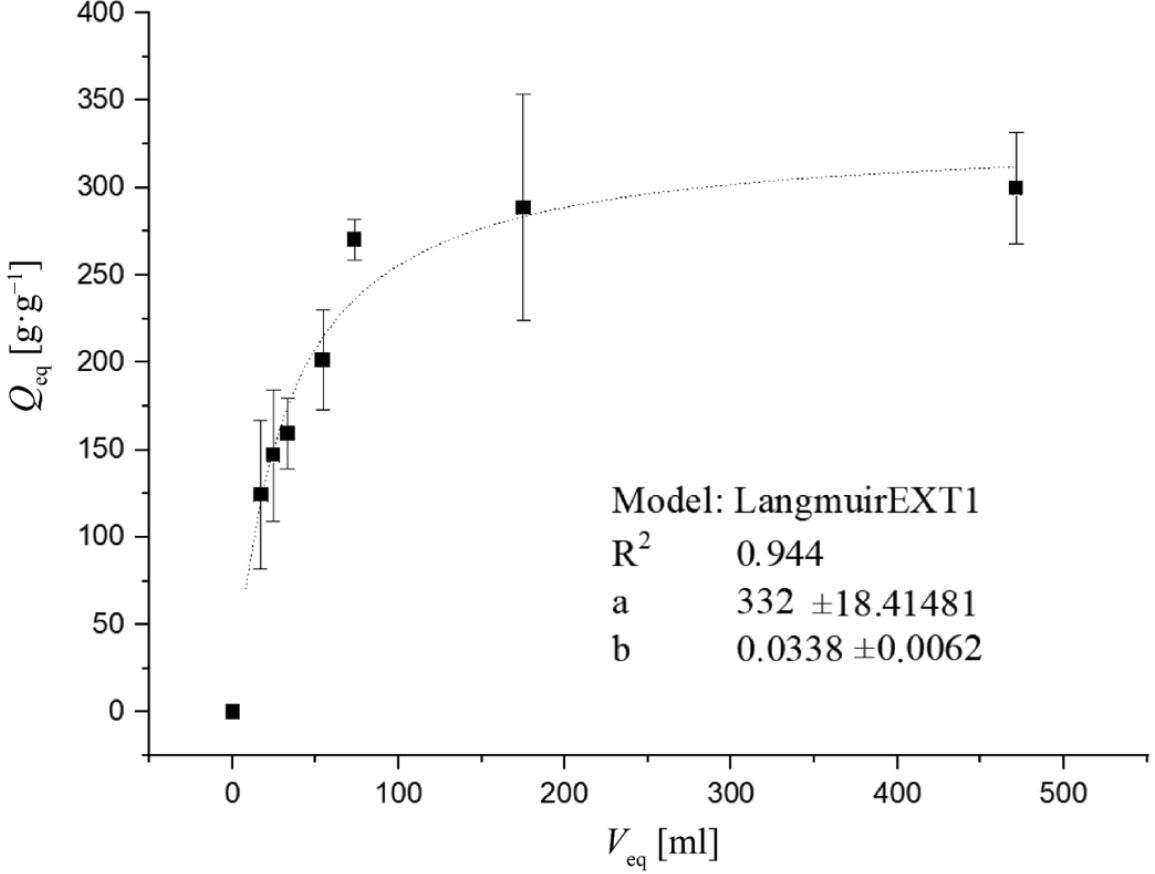
Dependence of equilibrium water absorption capacity (Qeq) on equilibrium volumes of unabsorbed water (Veq) described by Langmuir’s adsorption isotherm using a nonlinear regression method. The values represent the arithmetic means, and the error bars describe the standard deviation of three independent experiments
Source: own work.
The second experiment aimed to evaluate the kinetics of the hydrogel swelling process, specifically how the water absorption capacity of the SAP changes with the exposure time. The goal was to determine the optimal hydrogel swelling time of the polymer.
Based on Figure 3, it can be concluded that the hydrogel swelling process was most intense during the first 2 h of exposure, after which the hydrogel swelling capacity values changed only minimally. This suggests that the optimal hydrogel swelling time is 2 h. This conclusion was also supported by the results from the kinetics experiments using test tubes with a Microfil filter system. Consequently, a hydrogel swelling time of 2 h was applied in all subsequent experiments.
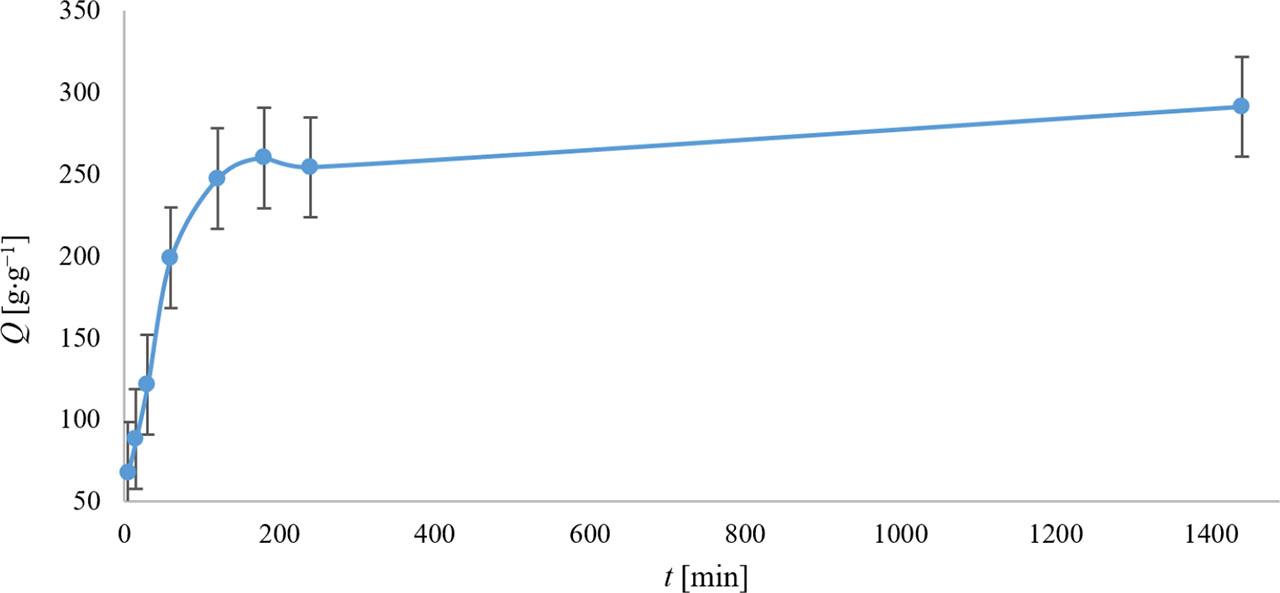
Dependence of SAP’s water absorption capacity (Q) on exposure time (t). The values represent the arithmetic means, and the error bars describe the standard deviation of three independent experiments
Source: own work.
As the second method for evaluating the kinetics of the hydrogel swelling process, the gravimetric method with the Microfil filter system was used.
From the curve described in Figure 4, it can be observed that the kinetics of hydrogel formation, whether in tea bags or test-tube experiments, followed a similar pattern, with intense hydrogel swelling during the first 2 h of exposure. After this period, the hydrogel swelling capacity increased only minimally. In this experiment, the maximum hydrogel swelling capacity (Q) after 2 h was recorded at 522 ±14 g⋅g−1, which was higher than in the tea bag experiment, where Q was only 247 ±14 g⋅g−1 after 2 h of exposure. The lower hydrogel swelling capacity observed in the tea bag method was attributed to the partial release of the hydrogel through the tea bag material, as previously mentioned.
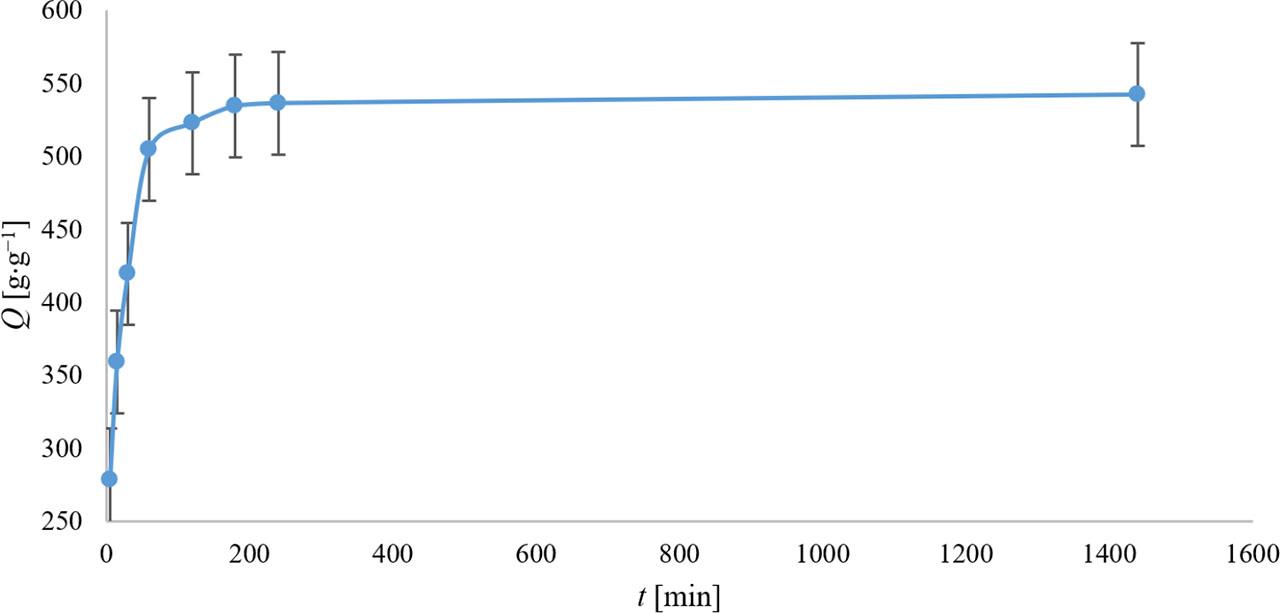
Dependence of SAP’s water absorption capacity (Q) on exposure time (t). The values represent the arithmetic means, and the error bars describe the standard deviation of three independent experiments
Source: own work.
A similar trend in the water absorption curve was also observed by Wang et al. (2024), who studied the kinetics of hydrogel formation for superabsorbent polymers. It is also noteworthy that the studied SAP exhibits relatively high-water absorption capacity compared to the starch-based SAPs used in similar experimental conditions, as demonstrated in the works of Lee et al. (2018), Lejcuś et al. (2018) and Chang et al. (2021).
To assess the stability of the hydrogel, expressed as the value of the water absorption capacity of SAP, determined in the presence of nutrients (individually or as complex Hoagland’s medium) or selected metals, test tube experiments were performed, where the hydrogel formation was evaluated gravimetrically.
Hoagland’s solution is a hydroponic nutrient medium that contains essential nutrients for plant growth and development, including N, P, K, Ca, Mg, S, and trace elements, such as Fe, Zn, Cu, and Mn. As shown in Figure 5, the formation of hydrogel significantly decreased even with a 10% diluted Hoagland’s medium. In the case of 100% Hoagland’s medium, hydrogel formation was minimal compared to water. This indicates that the components of Hoagland’s medium have a considerable impact on the ability of the SAP to bind water and form a hydrogel.
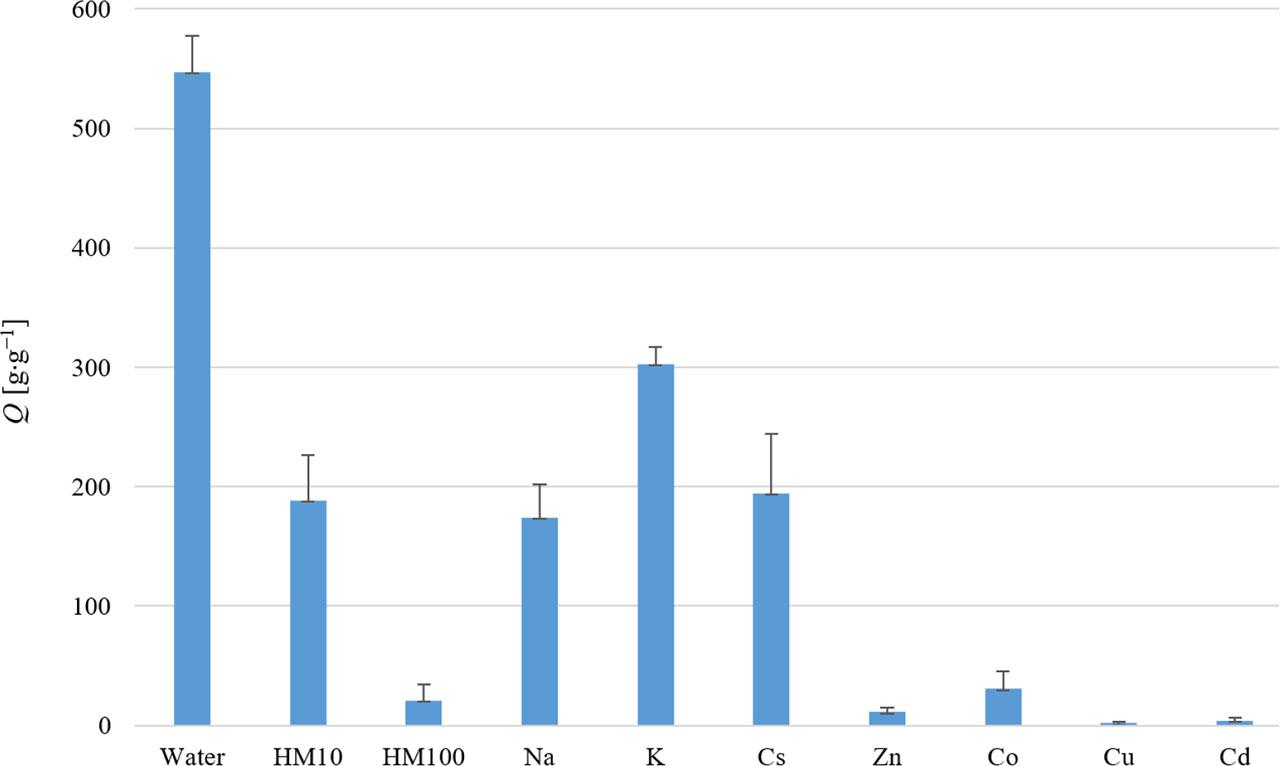
Water absorption capacity (Q) of SAP determined in the presence of Hoagland’s medium (10% and 100% strength) and metal ions with an equimolar concentration to the potassium content in the SAP. The values represent the arithmetic means, and the error bars describe the standard deviation of three independent experiments
Source: own work.
The potassium content in the SAP sample was determined using two analytical methods: flame photometry and scintillation gamma-spectroscopy. The measurements revealed that the potassium content in the analyzed SAP was 16.8%. To assess the hydrogel’s stability in the presence of metal ions with an equimolar concentration to the potassium content in the SAP, test tube experiments were conducted. The gravimetric assessment of hydrogel formation was performed using the Microfil filtration system.
As shown in Figure 5, the hydrogel formation decreased significantly in the presence of all the investigated ions. The hydrogel swelling capacity decreased by approx. half in the presence of K+ ions and by around two-thirds in the presence of Na+ and Cs+ ions. Minimal or no hydrogel formation was observed for the heavy metal ions studied. Mignon et al. (2019) explained that the osmotic pressure forces water into a polymer due to a higher ionic concentration inside the polymer compared to the surrounding solution because of the presence of charged and hydrophilic moieties onto the ionic monomers. The combination of these charged groups and additional polar moieties in a SAP (hydroxyl, carbonyl or amine functionalities) attracts water and induces hydrogen bonding. The amount of polar and/or ionic groups is directly proportional to the hydrogel’s swelling capacity. Introducing the SAP into a solution with a lower ionic concentration will lead to a higher hydrogel swelling capacity and, in the case of higher ion concentrations being present in the solution, the water absorption capacity of the SAP will be lower.
The experiments were focused on evaluating the effect of soil nutrients on the stability of superabsorbent polymers (SAPs) and their potential application in agriculture to improve water management, enhance crop resistance, and support agricultural production under the conditions of drought.
The first part of the study involved the physicochemical characterization of SAP as a mixture consisting of starch-based polymers and acrylic acid/acrylamide, including the assessment of its water absorption capacity, water binding kinetics, and optimization of experimental conditions.
In the second part, the effect of soil nutrients in the form of Hoagland’s hydroponic nutrient solution and various macro- and microelement solutions on the water absorption capacity of SAP was examined. Based on the experiments and analyses, the SAP exhibited relatively high-water absorption capacity Q = 522 ±14 g⋅g−1. However, a significantly reduced hydrogel swelling capacity was observed when Hoagland’s medium or metal ion solutions with an equimolar concentration to potassium were used. The hydrogel’s swelling capacity decreased by half in potassium (K) solutions and by two-thirds in sodium (Na) and cesium (Cs) solutions. Minimal hydrogel formation was observed in the presence of heavy metal ions with an equimolar concentration to potassium, and complete inactivation of the SAP occurred in the case of copper (Cu) solution.
The results of these experiments indicate that SAPs have potential applications in agriculture to enhance the efficiency of water use, increase drought tolerance in crops, and boost agricultural productivity. However, the composition of the soil solution or nutrient medium significantly affects the SAPs’ properties, particularly their water absorption capacity and stability. This must be considered when considering the use of SAPs in agricultural practices. It would be desirable for follow-up research in the field of evaluating the application of SAP to focus on finding new substances forming SAPs that meet the criteria of biodegradability and higher stability in soil in terms of maintaining their water absorption capacities.
