A promising noninvasive method to quantitatively study tissue alterations of human skin is electrical impedance spectroscopy (EIS). During an EIS measurement, an alternating current at varying frequencies is passed between electrodes and through the various layers of the skin. The resulting spectrum provides the skin tissue's overall resistance and reactance.
To aid in the interpretation of EIS spectra and to elucidate the mechanisms involved in EIS of human skin, mathematical models have been developed. Out of these models, equivalent-circuit constructs are the most common [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, ], where the electrical properties of the skin are estimated through a tissue-equivalent circuit comprising real and hypothetical electrical components. Mechanistic models based on the Maxwell equations and various degrees of resolution of the various skin layers have also been derived [11, 12, 13, 14, 15, 16,]. The key advantage of the mechanistic models is that they are based on actual physical phenomena in the form of conservation equations, as opposed to theoretical equivalent-circuit models. Their main disadvantages, however, are the higher computational cost and complexity in solving the constituent partial differential equation(s) with their constitutive relations and boundary conditions.
In light of the inherent disadvantages of a mechanistic model for EIS of human skin, our aims are threefold: (i) to elucidate the leading-order terms of our earlier mechanistic model [12], which has been shown to agree well with experimental measurements from clinical studies; (ii) to derive a reduced counterpart that brings with it significant computational savings whilst capturing the leading-order physics; and (iii) to secure approximate solutions of the skin impedance that are valid in the 1 kHz to 1 MHz frequency range we consider here and are as easy to employ as equivalent circuits.
The mathematical model comprising a Laplace equation in the frequency domain with varying material properties for the skin layers and boundary conditions for the EIS probe is first summarized together with the numerical method. We proceed to analyze the transport of alternating current through each of the layers – stratum corneum, viable skin and adipose tissue – of the skin as well as in the electrodes of the probe to uncover the leading order terms. In the following section, we discard lower order terms to secure a reduced mathematical model that retains the leading order physiochemical phenomena, which can be solved numerically at a significantly lowered computational cost. A Hankel transform then leads to approximate analytical solutions; these reductions and solutions are discussed and verified with the full set of equations (non-reduced model) for a large study with 7200 parameter combinations. Finally, conclusions are drawn and extensions of the mathematical model are highlighted in terms of higher resolution of the skin layers and various probe designs.
We start with the mathematical model for EIS measurements derived by Birgersson et al. [12], which treats the skin as a three-layer entity: stratum corneum (SC) as the outermost layer characterized by corneocytes; viable skin (VS) consisting of both living epidermis and dermis; and adipose tissue (AT) to account for the subcutis layer containing a high percentage of fat, as illustrated in Fig. 1a-b While the non-invasive EIS probe in the studies by Birgersson et al. [12] comprised four electrodes – one current detector (sense), one guard and two injects – we will here consider a simpler probe with similar dimensions but only one current detector (at I) and one voltage injector (at II). The dimensions and material properties of the electrodes and skin layers can be found in Table 1.
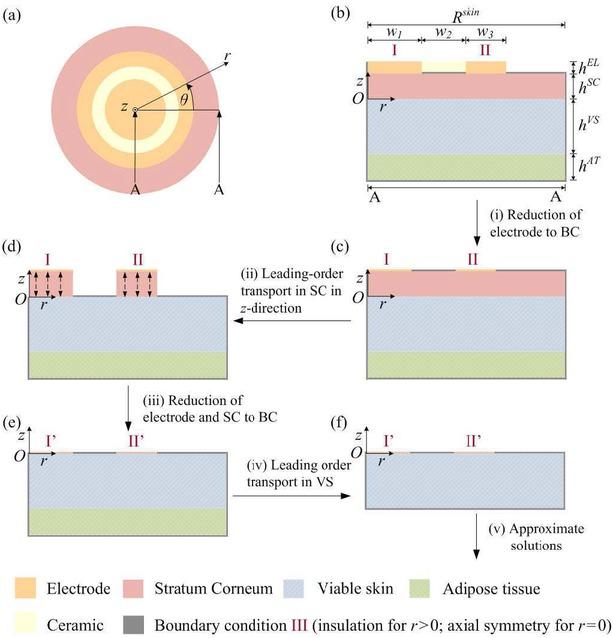
Schematic overview of the non-invasive two-electrode EIS probe and adjacent stratum corneum, viable skin and adipose tissue (a-b). The model reduction from the scaling analysis is illustrated in b-f.
Base-case dimensions and material parameters for the probe and skin layers.
| Current detection width, w1 | 2 mm | |
| Ceramic width, w2 | 1.2 mm | |
| Inject width, w3 | 1 mm | |
| Outer radius of the probe, R | 4.2 mm | |
| Outer radius of the skin, Rskin | 10 mm | |
| Electrode thickness, hEL | 0.1 mm | [18] |
| Stratum corneum thickness, hSC | 14 μm | [18] |
| Viable skin thickness, hVS | 1.2 mm | [18] |
| Adipose tissue thickness, hAT | 1.2 mm | [18] |
| Inject voltage, V0 | 0.05 V | |
| Electrical permittivity in vacuum, ɛ0 | 8.85 x107 | [19] |
| Fm-1 | ||
| Relative permittivity of electrodes, | 1 | [20] |
| Electrode conductivity, σEL | 4.56 x107 | [20] |
| Sm-1 | ||
The mathematical model can be written as
Here, the complex-valued phasors, Φ(r) and J(r) , for the potential and current density are functions of space only (although we note that they can be pulled back into the time domain), n is the unit normal vector pointing outwards to any given boundary, V0 is the applied voltage, and the location of the boundary conditions is given by roman numerals, see Fig. 1 for their location; the effective conductivity,
where σ k is the electric conductivity, ε0 and
The material properties for the three skin layers on the volar forearm are defined as follows [17]:
Here,
Coefficients for the conductivity and relative permittivity of the skin layers for 1 kHz to 1 MHz; the skin has been soaked with a 0.9% NaCl solution for 1 minute [17].
| j | ||
| 0 | -1.1803×101 | 1.7570×101 |
| 1 | 2.1404×101 | -1.7961×101 |
| 2 | -9.9955×100 | 8.5278×100 |
| 3 | 2.2537×100 | -2.0255×100 |
| 4 | -2.5509×10-1 | 2.3953×10-1 |
| 5 | 1.1516×10-2 | -1.1319×10-2 |
| j | ||
| 0 | 2.3688×101 | 6.7610×101 |
| 1 | -2.7471×101 | -7.0466×101 |
| 2 | 1.2952×101 | 3.2847×101 |
| 3 | -3.0088×100 | -7.7649×100 |
| 4 | 3.4167×10-1 | 9.2429×10-1 |
| 5 | -1. 5178×10-2 | -4.4465×10-2 |
| j | ||
| 0 | 1.7402×100 | 7.5844×100 |
| 1 | 0 | 8.3142×10-2 |
| 2 | 0 | -7.7214×10-1 |
| 3 | 0 | 1.1797×10-1 |
| 4 | 0 | -4.0926×10-4 |
| 5 | 0 | -5.2423×10-4 |
The impedance is defined as
with the total current measured at the sense, boundary I in Fig. 1b, defined as
We will refer to this model as the complete model.
We define the following dimensionless variables and dimensionless numbers:
Here, [ ] is the scale (absolute value), R = w1 + w2 + w3 is the overall radius of the outer electrode (inject) and
These dimensionless variables and numbers are substituted into the governing equations for each layer of the skin, which we shall consider separately. Between the different layers, we require continuity of the current density as well as potential – we do not explicitly state those boundary conditions in the coming analysis for the sake of brevity.
In the stratum corneum, we obtain the following dimensionless transport equations:
where
Here, ΠSC ~ 1, as can be inferred from Fig. 2, whence we can identify the current density scale, [J], from Eqs. 16 and 17 as
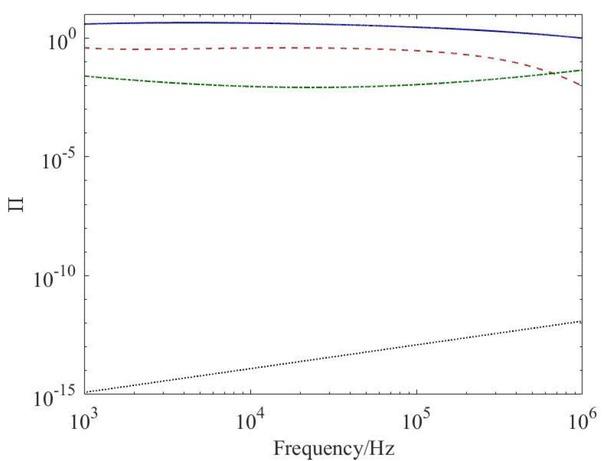
Dimensionless number, Π, for SC (–), VS (– –), AT (-⋅-) and EL (⋅⋅⋅).
Substituting the current density scale back into the governing equations, Eqs. 15, 16, 17, gives
where
Furthermore, the stratum corneum is slender with
R ~10-2 m and hSC ~10-5 m, such that
and
In other words, the current density in the radial direction is around a thousand times smaller than the current in the z-direction for the conditions and probe dimensions considered here, whence Eq. 18 reduces to
subject to the boundary conditions adjacent to the electrodes
and a continuous potential at the interface with the viable skin (N.B.: the potential drop in the electrodes is negligible at leading order as we shall show later). The current between the inject and the sense in the radial direction thus has to occur in the viable skin and/or adipose tissue, because the voltage is not enough to drive the current through the SC in the radial direction.
Returning to the current density scale for the frequency range of 1 kHz to 1 MHz that is of interest here, we find that
which provides the scale for the current density in the z-direction for the base-case parameters and conditions; in the radial direction, the scale for current density is
Finally, we confirm that scaling the potential,
potential drop underneath the sense compared to the inject originates from the fact that the area of the inject is roughly two times larger than the sense for the base case conditions.
For the viable skin,
where
and
In order for the current to be carried through the viable skin between the inject and the sense, we require that
as illustrated in Figure 3.
Dimensionless number,
This in turn suggests that we need to revisit the potential drop in the viable skin, which we assumed to be ~ V0 when we scaled Φ = Φ / V0 . If we rescale the potential,
with ΔΦVS denoting an as-of-yet unknown scale for the potential drop, Eqs. 27, 28, 29 take the form
with
Again, we require that
which gives the scale for the potential drop,
For the base case,
which agrees well with the numerical solutions of the complete model (not shown here).
For the adipose tissue,
where
and
Now, because
the current in the adipose tissue is mainly ohmic, such that we can write
For the current to be carried through the adipose tissue, we require that
Rescaling the potential drop like what we did for the viable skin,
and requiring
we find the scale for the potential drop in the adipose tissue,
With the base case parameters,
Clearly, the current from the probe cannot be carried through the adipose tissue at 1 MHz since it would require around forty times the applied voltage, V0; at the other end of the considered spectrum, 1 kHz, the voltage drop in the adipose tissue is around ten times larger than that of the viable skin; i.e.
In other words, most of the current from the inject would choose the easier path through the viable skin before even going deeper through the viable skin to reach the adipose tissue, after which the current would need to pass through the adipose tissue in the radial direction and then upwards through the viable skin again.
In the electrodes, the dimensionless governing equations can be written as
where
Because ΠEL ~ 10-15 -10-12 ≪ 1, the displacement current is negligible compared to the ohmic counterpart in the electrodes; in addition,
Now that we have analyzed the currents in the electrodes and three skin layers in detail to deduce the leading-order phenomena, we can combine the findings to arrive at a reduced model, as illustrated in Fig. 1b-1f
In the first step from Fig. 1b to 1c, the electrodes do not need to be resolved due to the negligible potential drops in these; we can thus move the boundaries for the ground, I, and applied potential, II, down to the stratum corneum; i.e. at the sense (I),
and at the inject (II),
where R1 = w1, R2 = w1 + w2 , R3 = w1 + w2 + w3 (= R) for notational convenience.
In the second and third steps from Fig. 1c to 1e, the leading-order transport in the stratum corneum for the frequencies considered here is in the z-direction, whence the PDE reduces to an ordinary differential equation, which we can integrate analytically and so reduce this layer to the modified boundary conditions, I’and II’ adjacent to the viable skin. We can capture these two steps in our reduced model by returning to Eq. 23 and writing it in dimensional form as
which gives us that J z is constant in the z-direction in the stratum corneum (the radial component is O(ΘSC ) ≪ 1 for the conditions we consider here); i.e., it is a function of r only. With the definition of the current density, we can write
which can be integrated once to give
With the boundary condition, Eq. 55, underneath the inject, we can determine the integration constant, C, and write the potential as
At the interface between the stratum corneum and viable skin (II'; z=0 in Eq. 59), we can now introduce the modified boundary condition in lieu of the stratum corneum and the inject as
where, again for convenience,
Returning to the sense (I) and the boundary condition, Eq. 54, we can repeat the integration above and write the modified boundary condition for the reduced model as
In the fourth step from Fig. 1e to 1f, the contribution of the adipose tissue to the impedance is negligible at leading order, whence we do not need to resolve this layer.
After these four steps, we can finally formulate the dimensional governing equations and boundary conditions, because it is not necessary to go through the entire nondimensionalization to employ the model:
We note that the initial boundary conditions – two Dirichlet and one Neuman condition – have been replaced by two Robin and one Neuman condition.
The reduced model – a Laplace equation with a Neuman and two Robin conditions – still requires a numerical method to solve. It is thus not as straight-forward to employ as equivalent circuits nor does it readily yield the functional form of the measured impedance and its dependence on the parameters. We therefore proceed with our analysis with a view to secure semi-analytical or analytical solutions (step v in Fig. 1). As we shall see, these solutions will be approximate in nature; i.e. we will make assumptions during the derivation.
Now, because we are primarily interested in the measured impedance (macroscopic, integral quantity) and not the pointwise potential distribution (microscopic quantity) throughout the electrodes and skin layers, we start by integrating the reduced model, Eqs. 63, 64, and invoking the divergence theorem,
where V is the volume and A is the outer area bounding the volume.
Introducing the boundary conditions, Eqs. 65, 67, into the integral formulation, Eq. 69, gives
where Фsen and Фinj are the area-averaged potentials at the sense and inject respectively:
Here, I is the total current and the areas of the sense and inject are
Let us define the overall potential drop across the viable skin as
and argue based on linearity that ΔV VS should be a function of the current density, the effective conductivity of the viable skin and a representative average length for the current flowing through the viable skin, L; i.e.
Assuming that we will later be able to quantify L, we now have four equations for our primary variables, Φ sen , Φinj , I, and Z:
After some algebra, we find the current and impedance as
with
Returning to the definition of S in Eq. 61, we can express C as
which acts as a dimensionless correction factor for the current and impedance; i.e. if
In particular, if we take Ainj ~ R2 and L - R , we see that
based on the potential drops that were defined in the earlier analysis for the skin layers. The condition
Let us now try to determine L and its functional form. For this purpose, we will carry out a Hankel transform of zeroth order, H0 { Φ ( r , z ) } , similar to Cheng et al. [21],
because it allows us to transform our axisymmetric Laplace equation in cylindrical coordinates (Eq. 63),
to an ordinary differential equation,
with the solution
where A(ξ ) and B(ξ ) are two functions that we will soon determine from the transformed boundary conditions; J0 is the 0th-order Bessel function of the first kind and Δ is the Laplace operator. At this stage, we note that our transformed solution automatically satisfies the circular symmetry and requires that
which is reasonable since we expect the measured impedance to mainly be determined by the current flowing between the two electrodes; in fact, we have in the original boundary condition, Eq. 67, artificially limited the computational domain by assuming insulation for r = Rskin and choosing Rskin sufficiently large not to affect the impedance significantly.
If we further assume that the current across each electrode is constant, we can simplify Eqs. 65 and 66 to
or, after the Hankel transform,
where we have used that the Hankel transform of zeroth order of a Heaviside function, U(a - r) , is defined as
with J1 and a denoting the 1st-order Bessel function of the first kind and a constant respectively. The remaining boundary condition, Eq. 67, becomes after transformation
Substituting the z-derivative of the solution, Eq. 89,
into Eq. 94 gives
Proceeding to the other boundary condition, Eq. 91, we find after substituting the solution thus far,
whence
and
With A(ξ) and B(ξ) in Eq. 89,
or
since
Taking the inverse Hankel transform,
we find the local potential distribution throughout the viable skin as
Note that the local potential, Υ(r, z) , at this stage is not the same as Φ(r, z) in our initial model with a Robin boundarycondition, Eqs. 65 and 66. We need to correct the potential distribution arising from the Hankel transform with the simplified boundary condition, Eq. 91, by shifting it with a constant, c; i.e.
The potential drop across the viable skin can now be found from
where
because
and
It is noteworthy that the integral on the right-hand-side (RHS) of Eq. 110 only depends on the lengths, R1, R2, R3 and hVS and not on the frequency. One thus only needs to solve the integral once for a given electrode design and thickness of the viable skin. Most importantly, we see that the functional form agrees with Eq. 76 and that
Substituting our expression for 1/ L into Eqs. 81, 82, 83 finally reveals the functional form for the current and impedance based on the Hankel transform (denoted with the subscript H) as
It is clear that this solution requires one numerical step for the integral, ℑ, in the correction factor, ℭH, whence we refer to it as a semi-analytical solution (a simple Matlab code can be found in Appendix A). With the current, IH, we can also calculate the average potentials at the sense and inject:
Let us determine the constant c; this can be done by introducing a reference potential, e.g. Φsen from 117 into Eq. 106 together with Eq. 108, such that
whence
If we want to identify the contributions from the individual layers, we can write the impedance as
or in its dimensionless form as
with the dimensionless numbers
The first two terms on the RHS in Eqs. 121 and 122 quantify the impedance contribution of the current passing through the stratum corneum underneath the sense and inject and the third term as it passes through the viable skin. In particular, the dimensionless form of the impedance reveals that the full model for the measured EIS can be reduced down to four dimensionless numbers Λ 2 , Λ 3 , ΠSC and ΠVS .
We solve both the complete mathematical model and the reduced counterpart in the finite-element solver COMSOL Multiphysics 5.2a [22]. The axisymmetric two-dimensional geometries of the complete, see Fig. 1b, and reduced model, see Fig. 1f, were resolved with around 104 and 500 mesh elements after a mesh-independent study to ensure mesh-independent solutions. The direct solver MUMPS was selected as the linear solver with a relative convergence tolerance of 10-6; and a typical run for one frequency required around 1 s (wall-clock time) with around 104 degrees of freedom on a quad-core 3.4 GHz workstation with 16 GB RAM for the complete model; the reduced model with 103 degrees of freedom was solved almost instantaneously (reported as 0 s in COMSOL). Additionally, the approximate semi-analytical and analytical solutions were calculated and post-processed in Matlab R2015 [23].
We have thus far derived a reduced model that only requires the numerical solution of a complex-valued Laplace equation in the viable skin with modified boundary conditions for the leading-order transport in the electrodes and stratum corneum. We have also been able to further reduce the computational complexity by securing approximate solutions for the impedance (a macroscopic property) as well as point-wise potential distribution (microscopic entity). To verify the fidelity of the reduced model as well as the approximate solutions, we will start by comparing these with the complete model at the macroscopic level and then at the microscopic level – here, verification refers to a numerical comparison. (The complete model was validated with experiments for two cohorts in an earlier study [17].)
At the macroscopic level, we have three expressions for the impedance:
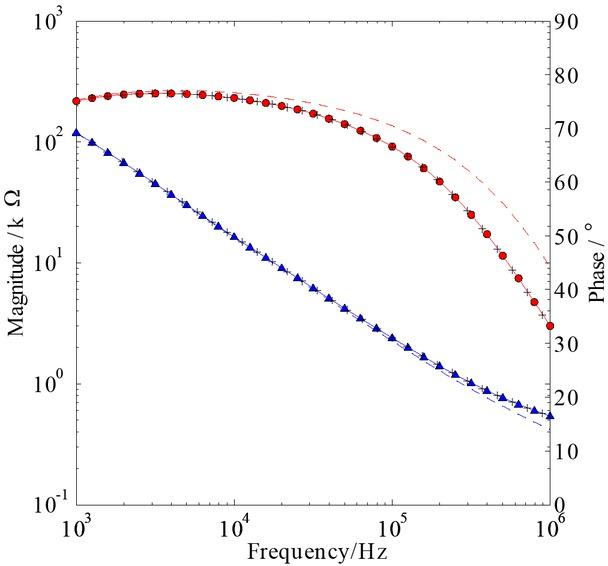
We compare their magnitudes and phases with the numerical solution of the complete model, Eqs. 1, 2, 3, 4, 5, for 1 – 103 kHz at base-case conditions (Table 1) in Fig. 4. Here, several features are apparent. First and foremost is that the reduced model, Zred, can predict the magnitude and phase of the EIS in the considered frequency range: the maximum relative error is less than 2% and 0.1% for the magnitude and phase respectively. For the base-case parameters, the key dimensionless number ΘSC = hSC / R ~ 10-3 ≪1 – the key condition for the reduced model to be valid. This condition should be fulfilled for most probe designs if the length scale, R ≪ hSC ~ 10-5 m. Second is that the magnitude and phase of the impedance predicted by the approximate solution from the Hankel transform, ZH, agrees well throughout the frequency range with a maximum relative error of around 2% and 1% for the magnitude and phase respectively. It agrees well despite our approximation of the current being constant underneath each electrode, which raises the question as to why this is.
The magnitude (▲) and phase (●) of the impedance from the numerical solution of the complete set of equations, the reduced set of equations, Zred (–); the Hankel counterpart, ZH (+); and the approximate analytical counterpart, Z₁ (– –).
The answer can be found in the stratum corneum, which acts as a significant resistance to the current and so evens out the current density profile underneath the electrodes – this is in particular the case around 1 kHz where
Parameters
Parameter combinations.
| ν | (1, 2.1, 4.6, 10, 22, 46)×103, (1, 2.2, 4.6, 10)×105 Hz |
| w1 | 0.5, 1, 2, 3 mm |
| w2 | 0.1, 0.5, 1.2, 2, 3 mm |
| w3 | 0.5, 1, 2, 3 mm |
| hSC | 8, 14, 20 μm |
| hVS | 0.8, 1.2, 1.6 mm |
To further test the fidelity of the reduced model, Zred, and approximate solution, ZH, let us compare it with the full set of equations for a set of parameters in Table 3 that correspond to different electrode designs with smaller and larger electrodes – w1, w2 and w3 – and thicknesses of stratum corneum, hSC, and viable skin, hVS, and frequencies, ν . All permutations of these parameters are considered for a total of 7200 parameter combinations. For the two skin layer thicknesses, we have taken the mean values ± two standard deviations [12]; i.e. 14±6 μm for stratum corneum and 1.2 ± 0.4 mm for viable skin.
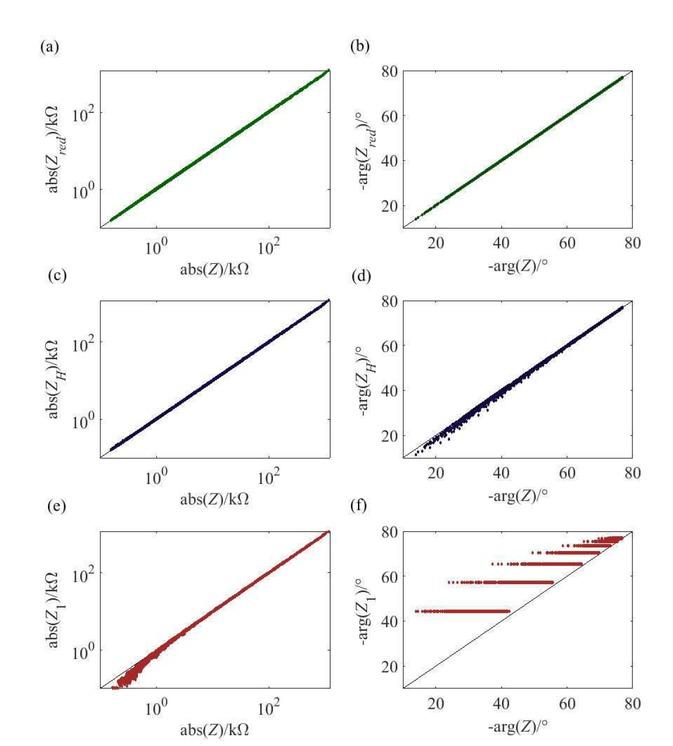
Starting with the reduced model in Fig. 5a-b, we see that it captures all the 7200 permutations of the parameters well with respect to the full model – the maximum relative error is 3% and 0.1% for the magnitude and phase respectively. For the approximate solution based on the Hankel transform, ZH, the picture is slightly different: It agrees well in terms of the predicted magnitude with a maximum relative error of 3% for all parameter combinations, but the error for its predicted phase increases for smaller angles and reaches around 24% for negative phase values of around 20o.
Verification of the magnitude and the phase of the approximate solutions with the full set of equations for the large parametric study: (a-b) reduced model, Zred, solved numerically; (c-d) solution, ZH, from the Hankel transform; (e-f) solution, Z₁, for ℭ≪1.
A closer inspection reveals that the specific parameter combinations for these predicted low negative phase values of around 20o are as follows: 1 MHz frequency, where the width of the sense, w1, is typically 3 mm, the width of the inject, w3, is 3 mm and the spacing between the electrodes, w2, is 1.2 - 2 mm, whilst hSC is either 8 or 1.4 μm (but not at the higher thickness of 2 μm) and hVS is at the lowest thickness of 0.8 mm. The key factors here are the large size of the electrodes (the inject is three times wider than in the base case) and the frequency of 1 MHz, for which
We also see that Z1 captures the measured magnitude reasonably well at high absolute values of the impedance but grows to a maximum relative error of around 70% at lower values of around 10 kΩ, whereas it only captures the phase close to 1 kHz (values in Fig. 5f close the diagonal) and then fails to describe the measured phase at frequencies larger than 1 kHz with a maximum relative error of around 220%. The latter is due to the fact that Z1 does not account for the current flow through the viable skin.
At the microscopic level throughout the viable skin, we can determine the point-wise potential distribution by solving the reduced model numerically or take the approximate solution arising from the Hankel transform of the reduced model together with the assumption of constant currents at the electrodes. The latter is given by
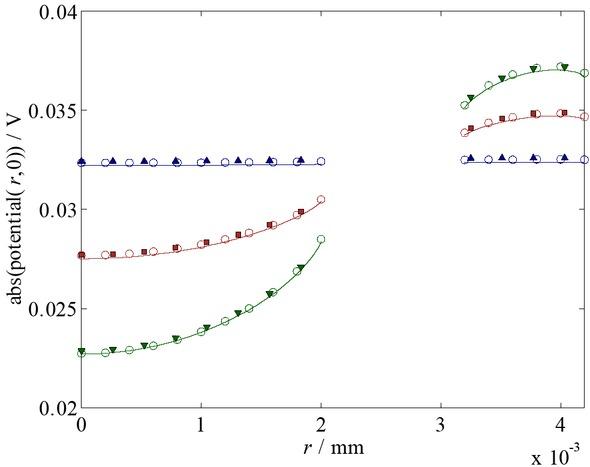
Let us first look at the potential distributions at the electrodes, as depicted in Fig. 6 for the base-case conditions.
The absolute value of the local potential distribution at z=0 m on the base case for the frequencies 1 kHz (▲), 0.328 MHz (■) and 1 MHz (▼); the reduced numerical model (–) and the Hankel counterpart (o). (N.B.: The verification points have been shifted slightly along r to prevent overlap.)
Again, we note the good agreement of the reduced model and approximate solution, Eq. 127, vis-à-vis the complete counterpart with a maximum relative error of around or less than 1%. Throughout the viable skin, the agreement remains good, as can be inferred from the visual comparison of the absolute value of the potential at 1 MHz in Fig. 7.
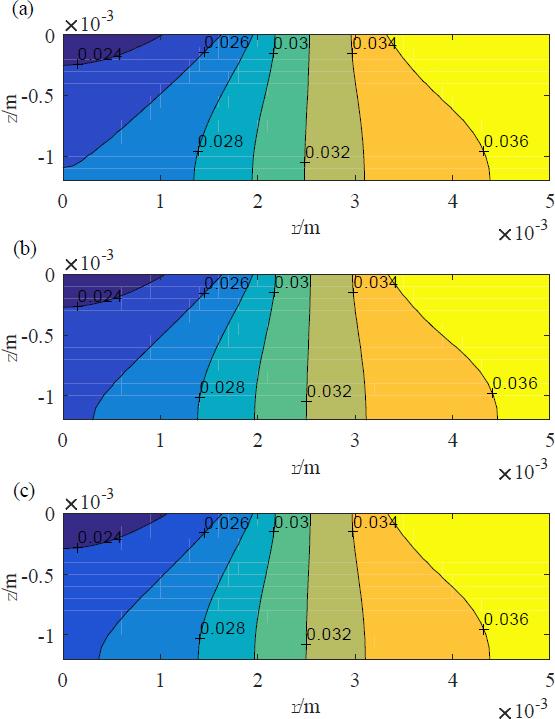
The absolute value of the potential distribution at 1 MHz for the base case in the viable skin: (a) the complete model; (b) the reduced model; and (c) the approximate solution from the Hankel transform.
Finally, we note that we could, although we do not do so here, also calculate and visualize the potential distribution in the stratum corneum by returning to the earlier analysis during which we reduced the stratum corneum to a boundary condition.
A scaling analysis of a mathematical model comprising a complex-valued Laplace equation with boundary conditions in the three outermost layers of a volar forearm – stratum corneum, viable skin and adipose tissue – for EIS measurements with a two-electrode probe has revealed the order-of-magnitudes of the current flow and potential drops and the dimensionless numbers that govern the measured impedance. These, in turn, have allowed for a reduction of the full set of equations (original model) to a reduced counterpart that only requires the numerical solution of the viable skin with modified boundary conditions that implicitly capture the contributions to the impedance from the electrodes, stratum corneum and adipose tissue. The reduced model was found to agree well with the complete model for the frequency range of 1 kHz to 1 MHz that is considered here.
Furthermore, a Hankel transform of the reduced model yielded a semi-analytical approximate solution that captures both the impedance and the point-wise potential distribution in the viable skin. Around 1 kHz, a closed-form expression was found as well. These solutions for the measured impedance are of similar functional form as the common equivalent circuits for EIS measurements of human skin and as easy to use; however, unlike equivalent circuits, which contain parameters to be fitted to experiments that are not always physical in nature, our solutions are based on the pointwise equations of change for charge transport, physical material properties and the probe design.
The presented mathematical model could be extended to include a higher resolution of the various layers that can be found in stratum corneum as well as viable skin. It can also easily be adjusted for modeling of other locations on the human body, different frequency ranges, as well as be modified for different probe designs with, e.g., rectangularshaped probes or more electrodes.