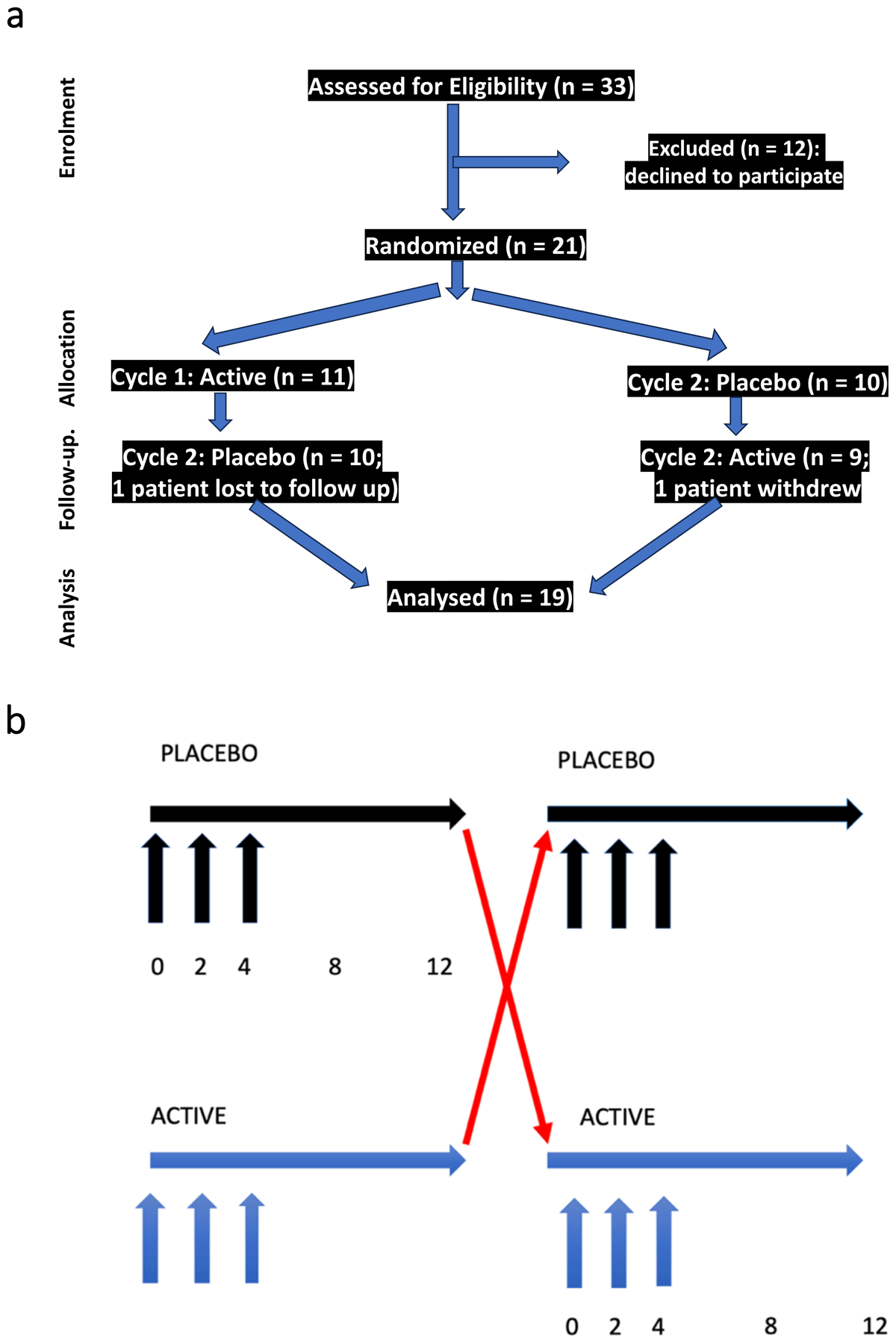
Figure 1
Trial Design.
Figure 1 summarizes the trial design. Figure 1a summarizes patient assessments by CONSORT criteria. 33 patients were assessed for eligibility, and 12 declined to participate (due to the possibility of receiving placebo). 21 patients were randomized in double blind fashion, 11 to active arm first followed by placebo, 10 to placebo arm first followed by active. One patient was lost to follow up and one patient withdrew). 19 patients completed both placebo and active arms, and all were included in the analysis. Figure 1b further illustrates the trial design. Patients were randomly assigned in double blind fashion to either active or placebo arm. Visits occurred at weeks 0 (enrollment), 2, 4, 8 and 12 in each segment (active or placebo). Injections were performed at weeks 0, 2 and 4 of both active and placebo segments.
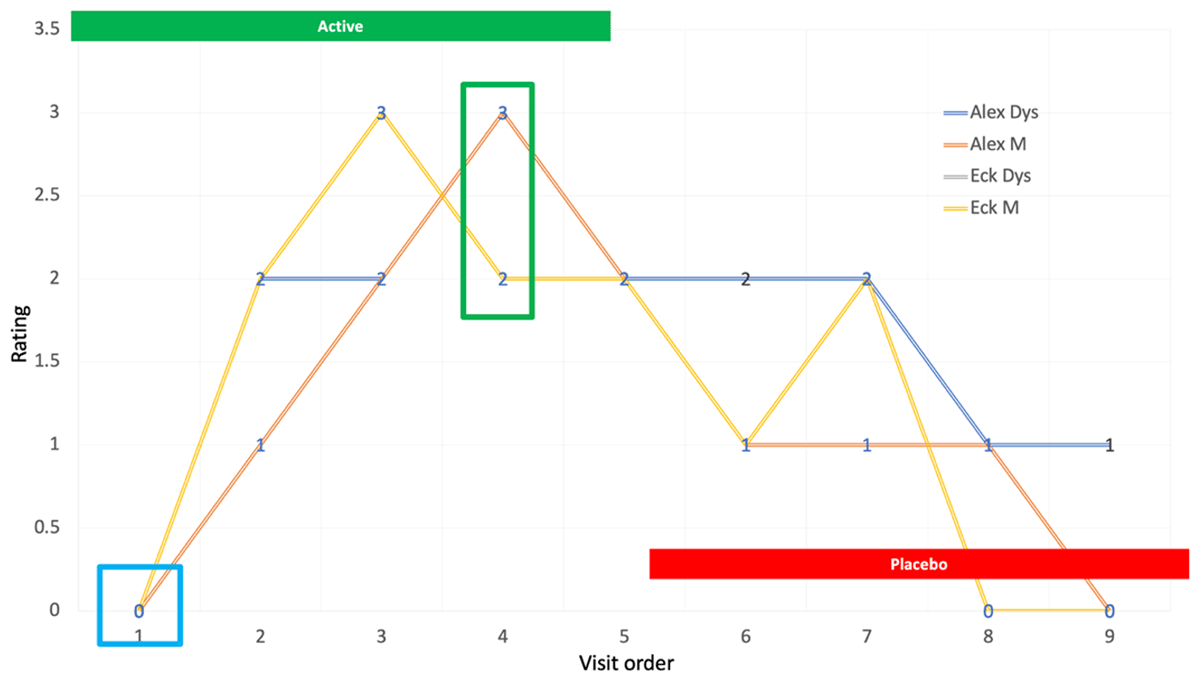
Figure 2
Graphic representation of ratings for a tabla player.
Blinded CGI ratings of musical (M) performance and dystonia severity (D) for a table player are depicted in Figure 2. After completion of the trial, it was determined that the patient had been assigned to the active arm first. Video segments from each visit appear in the accompanying video.
