Pancreatic ductal adenocarcinoma (PDAC) is the most common primary pancreatic neoplasm (Figure 1) and remains one of the most lethal malignancies, with survival strongly dependent on early detection and appropriate treatment strategies. Imaging plays a central role across all stages of patient management, from diagnosis and staging to the assessment of response to neoadjuvant therapy and post‑surgical surveillance.
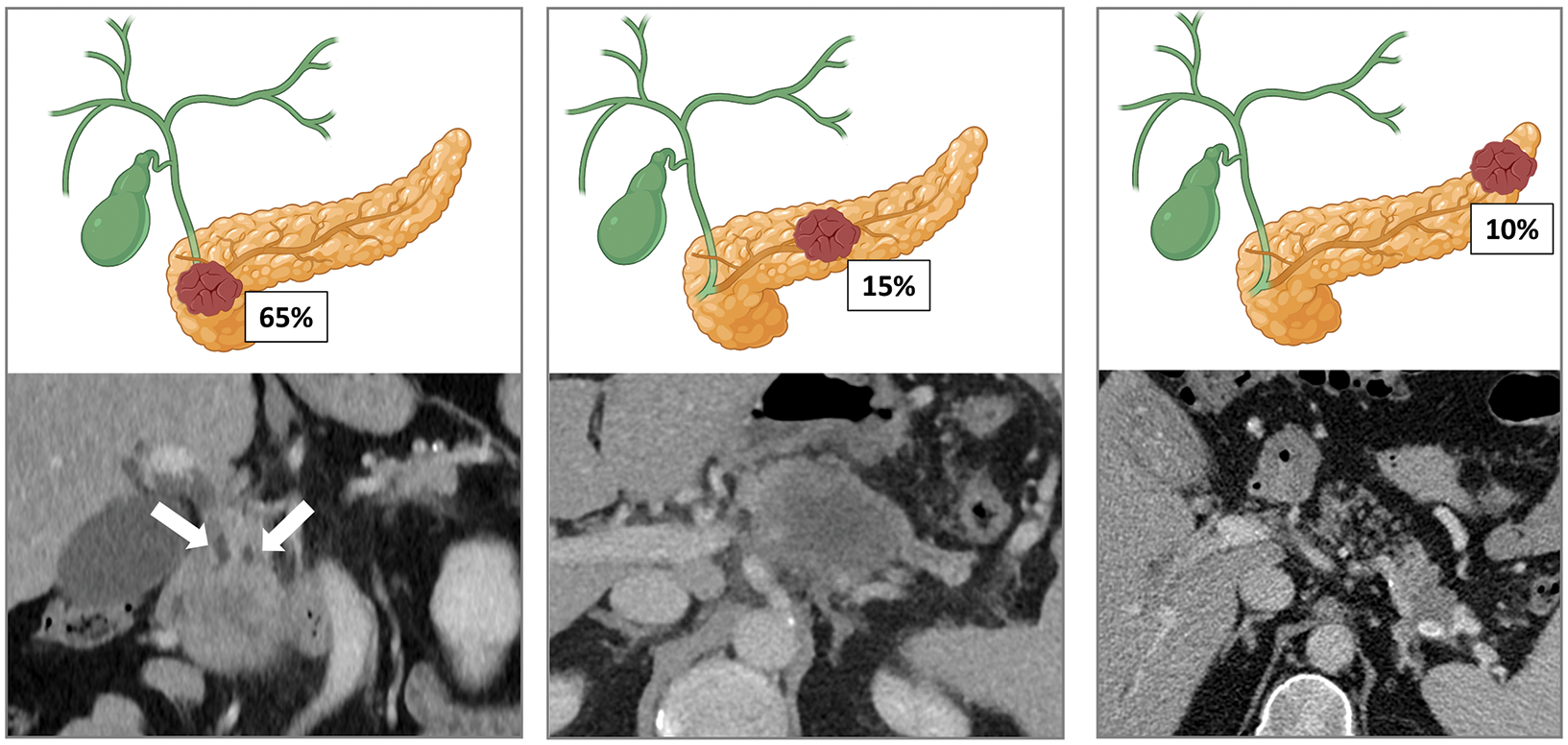
Figure 1
Approximately 65% of pancreatic adenocarcinomas are located within the head, 15% in the body and 10% in the tail. The remaining 10% are multifocal or diffuse. Tumors of the head often manifest earlier due to obstructive jaundice (with the characteristic “double duct sign”, as illustrated in our example). In contrast, tumors of the body and tail tend to be diagnosed at later stages and they are associated with a worse prognosis.
Contrast‑enhanced computed tomography (CT) is the principal imaging modality due to its high sensitivity for tumor detection and vascular assessment (Figure 2), while magnetic resonance imaging (MRI) is particularly useful for hepatic staging and problem‑solving in equivocal cases. Endoscopic ultrasound (EUS) complements cross‑sectional imaging by providing histological sampling with high diagnostic sensitivity.

Figure 2
Vascular assessment to define resectability status at diagnosis.
For disease staging, imaging is essential to define tumor resectability, usually classified as resectable, borderline resectable, locally advanced, or metastatic, according to vascular involvement, adjacent organ invasion, and distant spread. However, conventional imaging remains limited for lymph node assessment. In addition, an exclusive focus on anatomic criteria may overlook biological factors that are increasingly recognized as key for prognosis and treatment planning. In this setting, structured reporting has been recommended to provide standardized communication and support multidisciplinary management.
Evaluating treatment response after neoadjuvant therapy remains a major challenge. Tumor size alone is not a reliable marker due to persistent fibrotic stroma and the impossibility for differentiating post‑treatment fibrosis from viable tumor tissue, making biochemical markers and stability of disease on imaging the main indicators for resectability. Advanced modalities, including diffusion‑weighted MRI, positron emission tomography (PET), and emerging molecular imaging techniques, show promising results but require further validation.
Following surgical resection, surveillance strategies are still debated, although regular imaging and tumor marker follow‑up appear to improve early recurrence detection and patient outcomes.
In conclusion, imaging is crucial in the management of PDAC; however, important limitations persist in staging, assessing treatment response, and establishing standardized follow‑up protocols. Future integration of advanced imaging techniques and artificial intelligence may enhance diagnostic accuracy, guide personalized treatment decisions, and ultimately improve patient survival.
Competing Interests
The authors have no competing interests to declare.
