Figure 1
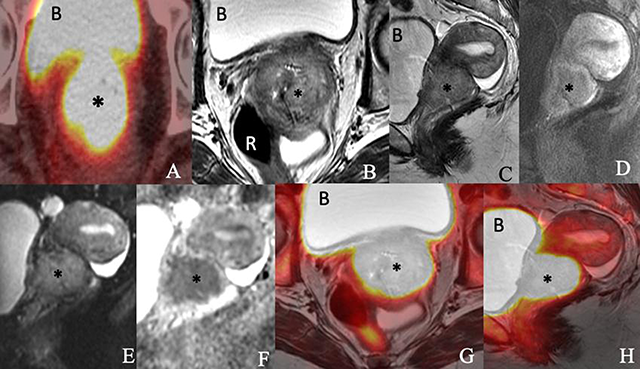
Images of a 76-year-old woman with newly diagnosed squamous cell carcinoma of the cervix consisting of an (A) axial PET/CT, (B) axial T2-weighted, (C) sagittal T2-weighted, (D) sagittal postcontrast T1-weighted, (E) sagittal diffusion-weighted (F) sagittal apparent diffusion coefficient map of MRI, (G) axial T2-weighted PET/MRI and (H) sagittal T2-weighted PET/MRI images show a 5.4 × 4.6 × 4.1-cm enhancing FDG-avid cervical mass (*) invading the parametrium and extending into the vaginal fornices and lower uterine segment. The mass exhibits restricted diffusion. The bladder (B) and rectum (R) appear to be uninvolved.
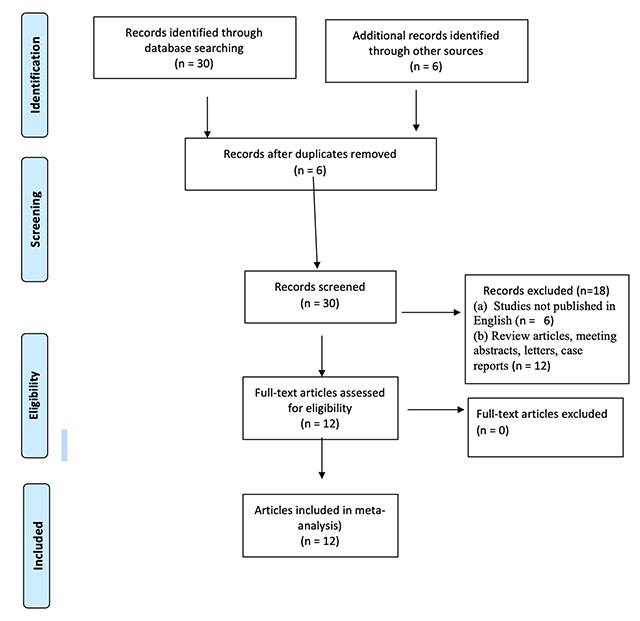
Figure 2
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the meta-analysis.
Table 1
Characteristics of the 12 Studies of PET/MRI Performed in the Same Patient Population.
| Reference | Year | Country | PET/MRI or Fusion | Study Type | Number of Subjects | Primary Finding | Objective |
|---|---|---|---|---|---|---|---|
| Kim et al. [5] | 2009 | Republic of Korea | Signa 1.5T system (GE Healthcare, Milwaukee, WI) Image fusion: Advantage Workstation (version 4.3; GE Healthcare) | Retrospective | 79 | Cervical cancer (n = 79) | Staging |
| Fiaschetti et al. [6] | 2011 | Italy | 3T permanent magnet (Achieva; Philips, Best, Netherlands) Image fusion: Advantage MR-PET Fusion on Advantage Workstation (version 4.4; GE Healthcare) | Prospective | 24 | Ovarian lesions: malignant (n = 19), benign (n = 5) | Staging |
| Kitajima et al. [7] | 2013 | Japan | 1.5T MR scanner (Signa EchoSpeed Plus Excite 1.5T; GE Healthcare) Image fusion: Advantage Workstation (version 4.5; GE Healthcare) | Retrospective | 30 | Endometrial cancer (n = 35) | Staging |
| Kitajima et al. [9] | 2014 | Japan | 1.5T MR scanner (Achieva; Philips) Image fusion: Advantage Workstation (version 4.5; GE Healthcare) | Retrospective | 35 | Cervical cancer (n = 35) | Staging |
| Kitajima et al. [10] | 2014 | Japan | 1.5T MR scanner (Achieva; Philips) Image fusion: Advantage Workstation (version 4.5; GE Healthcare) | Retrospective | 30 | Locally recurrent disease (n = 16), pelvic lymph node metastases (n = 8), bone metastases (n = 3), peritoneal dissemination (n = 5) | Recurrence and metastatic disease |
| Grueneisen et al. [8] | 2014 | Germany | 3T PET/MRI Biograph scanner (Siemens Healthineers, Erlangen, Germany) | Prospective | 48 | Primary cancer (n = 27), recurrence (n = 21) | Staging and recurrence |
| Queiroz et al. [13] | 2015 | Switzerland | 3T Discovery MR750w (GE Healthcare) Image fusion: Advantage Workstation (version 4.5; GE Healthcare) | Prospective | 26 | Ovarian (n = 12), cervical (n = 7), endometrial (n = 4), vulvar (n = 1), and primary peritoneal (n = 1) cancer and uterine metastasis (n = 1) | Staging |
| Grueneisen et al. [12] | 2015 | Germany | 3T PET/MRI Biograph scanner (Siemens Healthineers) | Prospective | 24 | Ovarian (n = 13), cervical (n = 7), and endometrial (n = 4) cancer | Recurrence |
| Grueneisen et al. [11] | 2015 | Germany | 3T PET/MRI Biograph scanner (Siemens Healthineers) | Prospective | 27 | Primary cervical cancer (n = 27) | Staging |
| Stecco et al. [14] | 2016 | Italy | 1.5T MRI scanner (Achieva Intera; Philips) Image fusion: Leonardo multimodality workstation (Siemens Healthineers) | Retrospective | 27 | Cervical (n = 14) and endometrial (n = 13) cancers | Staging |
| Kirchner et al. [15] | 2017 | Germany | 3T PET/MRI scanner (Biograph mMR; Siemens Healthineers) | Prospective | 43 | Ovarian (n = 23), cervical (n = 12), endometrial (n = 4), vulvar (n = 3), and vaginal (n = 1) cancers | Recurrence |
| Mongula et al. [16] | 2018 | Netherlands | 3T PET/MRI scanner (Biograph mMR; Siemens Healthineers) | Prospective | 10 | Cervical cancer (n = 10) | Response assessment after radiation therapy |
Table 2
Tabular presentation of QUADAS-2 results of the selected articles.
| Study | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Kim et al. [5] | ☺ | ☹ | ☺ | ? | ☺ | ☺ | ☺ |
| Fiaschetti et al. [6] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Kitajima et al. [7] | ☺ | ☺ | ☺† | ? | ☺ | ☺ | ☺ |
| Kitajima et al. [9] | ☺ | ☺ | ☺† | ? | ☺ | ☺ | ☺ |
| Kitajima et al. [10] | ☺ | ☺ | ☺† | ? | ☺ | ☺ | ☺ |
| Queiroz et al. [13] | ☺ | ☺ | ☺† | ? | ☺ | ☺ | ☺ |
| Grueneisen et al. [12] | ☺ | ☺ | ☺† | ? | ☺ | ☺ | ☺ |
| Grueneisen et al. [8] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Grueneisen et al. [11] | ☺ | ☹ | ☺ | ? | ☺ | ☺ | ☺ |
| Stecco et al. [14] | ☺ | ☺ | ☺ | ? | ☺ | ☺ | ☺ |
| Kirchner et al. [15] | ☺ | ☺ | ☺† | ? | ☺ | ☺ | ☺ |
| Mongula et al. [16] | ☺ | ☺ | ☹ | ? | ☺ | ☺ | ☺ |
[i] † Reference standards included histopathology and imaging follow up.
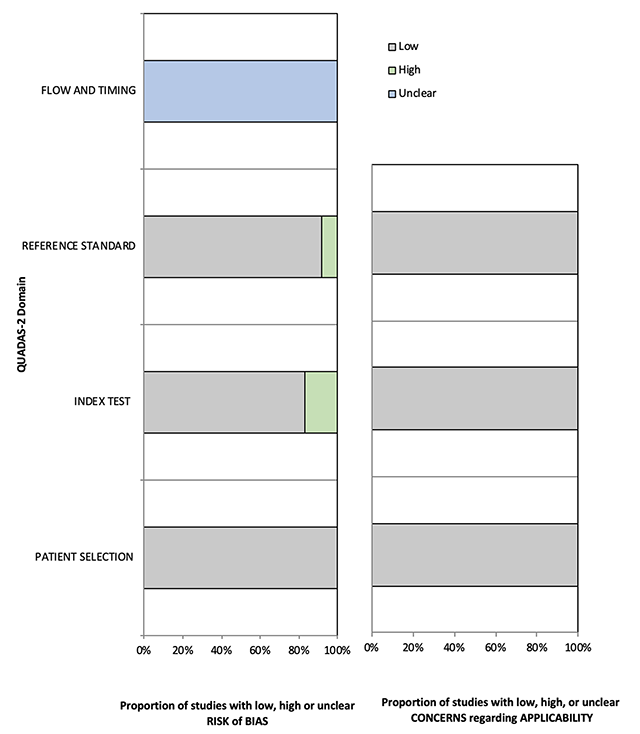
Figure 3
Methodological quality of all eligible studies according to QUADAS-2.
Table 3
Diagnostic Performance of PET/MRI in Imaging of Gynecological Malignancies (Patient-Based Analysis).
| Parameter | PET/MRI | 95% CI |
|---|---|---|
| No. of TP results | 179 | – |
| No. of TN results | 346 | – |
| No. of FP results | 30 | – |
| No. of FN results | 45 | – |
| Sensitivity (%) | 74.2 | 66.2–80.8 |
| Specificity (%) | 89.8 | 82.2–94.3 |
| DOR | 26 | 10–67 |
| AUC | 0.834 | – |
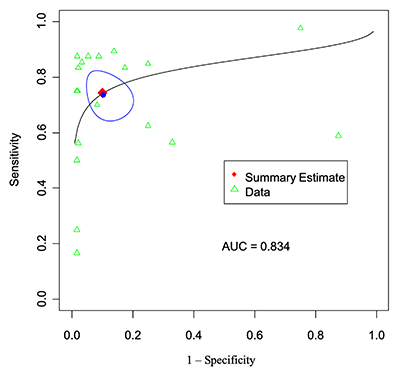
Figure 4
Patient-level analysis: SROC curve for PET-MRI.
Table 4
Diagnostic Performance of PET/MRI in Imaging of Gynecological Malignancies (Lesion-Based Analysis).
| Parameter | PET/MRI | 95% CI |
|---|---|---|
| No. of TP results | 496 | – |
| No. of TN results | 730 | – |
| No. of FP results | 70 | – |
| No. of FN results | 67 | – |
| Sensitivity (%) | 87.5 | 75.8–94.0 |
| Specificity (%) | 88.2 | 84.2–91.3 |
| DOR | 50 | 23–111 |
| AUC | 0.922 | – |
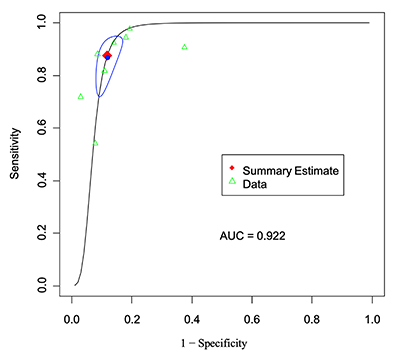
Figure 5
Lesion-level analysis: SROC curve for PET-MRI.
