Table 1
Inclusion and exclusion criteria.
| INCLUSION CRITERIA | EXCLUSION CRITERIA |
|---|---|
| Study concerns patients with at least one of the selected chronic illnesses (COPD, diabetes, depression, CHF, chronic kidney disease, or dementia) OR explicitly focuses on patients with multimorbidity | No full text available (conference abstract, poster presentation) |
| The payment reform under study explicitly targets patients with multimorbidity and/or introduces a payment structure that can be beneficial for patients with multimorbidity by stimulating integrated care | The study is not about a payment reform (e.g. organisational reform only) |
| Peer-reviewed study, retrospective and prospective (e.g. quasi-experimental study; RCT) | The payment reform does not stimulate the integrated delivery of care to the patients in that it does not comply with our definition of a targeted payment reform |
| Outcomes concern both the quality and utilisation of healthcare | The outcomes of the study concern only the quality or utilisation of healthcare |
| Published since 01/01/2000 | No original data |
| Written in English, Dutch |
[i] COPD: Chronic obstructive pulmonary disease, CHF: Chronic heart failure.
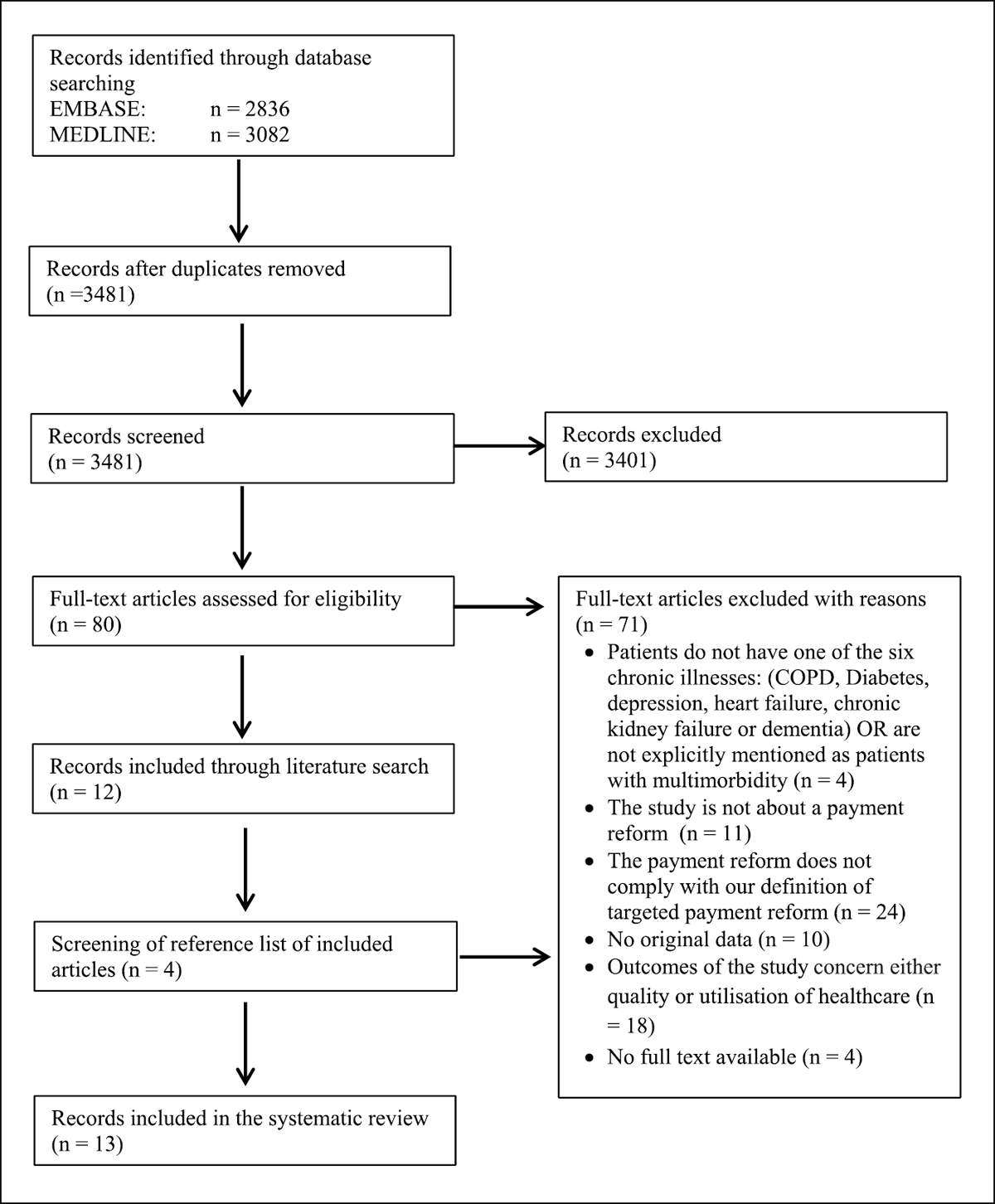
Figure 1
PRISMA flow diagram.
Table 2
Study characteristics.
| STUDY & COUNTRY [REFERENCE] | TARGET POPULATION | INTERVENTION | PROGRAMME CONTENTS | N (INTERVENTION, CONTROL)*1 | SETTING | DATA COLLECTION PERIOD | STUDY TYPE (ANALYSES) | OUTCOMES |
|---|---|---|---|---|---|---|---|---|
| Bhatt, S.P. United States [28] | COPD | Bundle*2 | Post-acute care bundle: antibiotics, educational materials, interval follow-up, and periodic phone calls | 78, 109 | Secondary (hospital) care | 2012 vs. 2014 | Cohort study with control group (independent sample statistical tests) | HR, EDV, Vis, HC, LoS |
| Koehler, B.E. United States [29] | PwM | Bundle | Elderly care bundle designed as an intensive patient-centred educational programme. Includes daily visits during hospital stay, standardised phone calls for follow-up appointments and education, and medication verification post discharge | 22,20 | Secondary (hospital) care | 2007 | Randomised control trial (independent sample statistical test) | HR, LoS |
| Morton, K. United Kingdom [30] | COPD | Bundle | COPD discharge bundle: technique (inhalers), action plan, pulmonary rehabilitation, smoking cessation, and specialist follow up. | 4657, 4515 | Secondary (hospital) care | 2013–2017 | Pre-post study with control group (regression models) | Mor, HR, EDV, LoS |
| Parekh, T.M. United States [31] | COPD | Bundle*2 | Post-acute care bundle: expedited follow-up visits in a COPD focused clinic, home calls, medication assistance, and tobacco cessation counselling. | 459, 239 | Secondary (hospital) care | 2012–2014 | Cohort study with control group (independent sample statistical tests) | Mor, HR, EDV, HC, LoS |
| Pawaskar, M. United States [32] | Diabetes | Capitation | Managed care organisations receive a fixed amount of payment per enrolee per month | 3763, 4818 | Primary care, secondary (hospital) care | 1999–2005 | Cohort study with control group (regression models) | ADU, Hos, EDV, Vis |
| Quinn, A.E. Canada [33] | Diabetes, CKD | Capitation | A salary-like payment that covered clinical, research, and teaching time | 15949, 15949 | Secondary (hospital) care | 2011–2015 | Cohort study with matched control group (regression) | DRE, Hos, EDV, Vis, HC |
| Joynt Maddox United States [21] CHF, COPD | DRG*2 | BPCI-model 2 bundle: Participating hospitals assume accountability for the costs of all care within 30, 60, or 90 days after hospitalisation for one or more of 48 conditions | 226, 407 hospitals | Secondary (hospital) care | 2013–2015 | Pre-post study with matched control group (regression models) | Mor, HR, EDV, HC, LoS | |
| Kutz, A. Switzerland [34] | COPD | DRG | All costs related to all acute inpatient hospital services | 19046, 30764 | Secondary (hospital) care | 2009–2015 | Pre-post study without control group (regression models) | Mor, HR, LoS |
| Lichkus, J. United States [35] | CHF | DRG*2, 3 | One bundled payment per 90-day episode of care initiated by an anchor admission for CHF exacerbation. | 283, 316 | Secondary (hospital) care | 2013–2017 | Pre-post study without control group (t-tests) | HR, HC |
| Maughhan, C. United States [36] | Dementia | DRG*2 | BPCI-model 2 bundle: Participating hospitals assume accountability for the costs of all care within 30, 60, or 90 days after hospitalisation for one or more of 48 conditions. | 45007, 45007 episodes | Secondary (hospital) care | 2011–2012 & 2013–2016 | Pre-post study with matched control group (regression models) | Mor, HR, EDV |
| Salzberg, C.A. United States [37] | Diabetes | Global budget*3 | Primary care practices receive a monthly, risk-adjusted total payment for the comprehensive care of all patients in the practice | 64471, 133345 | Primary care | 2008–2013 | Pre-post study with matched control group (regression models) | HR, Hos, EDV, Vis, HC |
| Cross, D.A. United States [38] | PwM | P4P | P4P-programme with incentives linked to 1) Medical Home Practice Transformation, 2) Provider-delivered Care Management, and 3) Practice Quality Assessment | 17501, 195344 | Primary care | 2010–2013 | Cohort study with control group (regression models) | DRE, ADU, HR, Hos, EDV, Vis, HC |
| Hollander, M.J. Canada [39] | Diabetes, CHF, COPD | P4P | P4P-programme with incentives linked to the provision of guidelines-based care to patients with chronic conditions | 176542, 209064 | Primary care | 2010–2011 | Cohort study with matched control group (paired samples t-test) | HR, Hos, HC |
[i] *1 Total number included in analyses relevant to this study – patients unless indicated otherwise.
*2 Part of Bundled Payments for Care Improvement (BPCI) Initiative.
*3 Payment reform is accompanied by an organisational reform.
COPD: Chronic obstructive pulmonary disease, PwM: Patients with multimorbidity, CHF: Chronic health failure, CKD: Chronic kidney disease.
DRE: Disease related examination(s)/treatment(s), ADU: Appropriate drug use, Mor: Mortality, HR: Hospital readmissions, Hos: Hospitalisations, EDV: Emergency Department Visits, Vis: Visits, HC: Healthcare costs, LoS: Length of Stay.
Table 3
Effects of targeted payment reforms on the quality of care outcomes.
| STUDY | PAYMENT MODEL | DISEASE-RELATED EXAMINATION(S)/TREATMENT(S) | APPROPRIATE DRUG USE | MORTALITY | HOSPITAL READMISSIONS | RISK OF BIAS | |
|---|---|---|---|---|---|---|---|
| AC | DR | ||||||
| Bhatt, S.P. | Bundle | n.a. | n.a. | n.a. | None | None | Critical |
| Koehler, B.E. | Bundle | n.a. | n.a. | n.a. | Mixed | n.a. | Some concerns |
| Morton, K. | Bundle | n.a. | n.a. | None | None | None | Moderate |
| Parekh, T.M. | Bundle | n.a. | n.a. | Decrease | None | n.a. | Serious |
| Pawaskar, M. | Capitation | n.a. | Decrease | n.a. | n.a. | n.a. | Serious |
| Quinn, A.E. | Capitation | None | n.a. | n.a. | n.a. | n.a. | Moderate |
| Joynt Maddox, K.E. | DRG | n.a. | n.a. | None | None | n.a. | Serious |
| Kutz, A. | DRG | n.a. | n.a. | None | None | n.a. | Moderate |
| Lichkus, J. | DRG*1 | n.a. | n.a. | n.a. | None | n.a. | Critical |
| Maughhan, B.C. | DRG | n.a. | n.a. | None | None | n.a. | Moderate |
| Salzberg, C.A. | Global budget *1 | n.a. | n.a. | n.a. | None | n.a. | Moderate |
| Cross, D.A. | P4P | Increase | Increase | n.a. | Decrease | n.a. | Moderate |
| Hollander, M.J. | P4P | n.a. | n.a. | n.a. | None | n.a. | Serious |
[i] AC: All-cause, DR: Disease-related, n.a. Not applicable.
*1 Payment reform is accompanied by an organisational reform.
Increase’ or ‘decrease’ signifies that the study found a significant (p ≤ 0.05) effect in all outcomes related to a specific outcome domain. ‘Mixed’ was used for studies with varying outcomes within one domain. ‘None’ was used for studies that found no statistically significant effect (p ≤ 0.05) for any of the outcomes related to a specific outcome domain or that studies did not report on any significance.
Table 4
Effects of targeted payment reforms on outcomes related to the utilisation of healthcare.
| STUDY | PAYMENT MODEL | HOSPITALISATIONS | ED VISITS | VISITS | HEALTHCARE COSTS | LENGTH OF STAY | RISK OF BIAS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | DR | AC | DR | AC | DR | AC | DR | ||||
| Bhatt, S.P. | Bundle | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | None | n.a. | n.a. | Critical |
| Koehler, B.E. | Bundle | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | None | Some concerns |
| Morton, K. | Bundle | n.a. | n.a. | n.a. | None | n.a. | n.a. | n.a. | n.a. | None | Moderate |
| Parekh, T.M. | Bundle | n.a. | n.a. | Decrease | n.a. | n.a. | n.a. | Decrease | n.a. | Decrease | Serious |
| Pawaskar, M. | Capitation | Increase | n.a. | Increase | n.a. | Decrease | n.a. | n.a. | n.a. | n.a. | Serious |
| Quinn, A.E. | Capitation | None | n.a. | None | n.a. | None | n.a. | None | n.a. | n.a. | Moderate |
| Joynt Maddox, K.E. | DRG | n.a. | n.a. | None | n.a. | n.a. | n.a. | None | n.a. | None | Serious |
| Kutz, A. | DRG | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | None | Moderate |
| Lichkus, J. | DRG*1 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | None | n.a. | n.a. | Critical |
| Maughhan, B.C. | DRG | n.a. | n.a. | None | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Moderate |
| Salzberg, C.A. | Global budget *1 | Increase | n.a. | None | n.a. | None | n.a. | None | n.a. | n.a. | Moderate |
| Cross, D.A. | P4P | Increase | n.a. | None | n.a. | None | n.a. | None | n.a. | n.a. | Moderate |
| Hollander, M.J. | P4P | None | n.a. | n.a. | n.a. | n.a. | n.a. | Increase | n.a. | None | Serious |
[i] ED: Emergency Department, AC: All-cause, DR: Disease-related, n.a. Not applicable.
* 1 Payment reform is accompanied by an organisational reform.
Increase’ or ‘decrease’ signifies that the study found a significant (p ≤ 0.05) effect in all outcomes related to a specific outcome domain. ‘Mixed’ was used for studies with varying outcomes within one domain. ‘None’ was used for studies that found no statistically significant effect (p ≤ 0.05) for any of the outcomes related to a specific outcome domain or that studies did not report on any significance.
