Table 1
Key stages and procedures used in conducting this scoping review.
| Stage | Description |
|---|---|
| 1. Clarifying purpose and identifying research questions | • Key research questions were shared with the expert panel and questions were refines to balance breadth with feasibility |
| 2. Identifying relevant studies | • Development and refinement of search strategies and selection of databases |
| • Testing and refinements of inclusion and exclusion criterion for screening | |
| 3. Study selection | • Independent application of screening criterion at two levels – title and abstract review and full article review by two reviewers (AIK and EA) |
| • Resolution of disagreements by a third reviewer (VK) to determine final inclusion/exclusion | |
| 4. Data extraction | • Development, testing and application of the data extraction tool |
| 5. Data analysis | • Summarizing descriptive characteristics of included articles |
| • Thematic analysis of extracted data and assessing the implications of findings for future research and policy changes | |
| 6. Consultation with key stakeholders | • Development of a knowledge translation strategy to share the overall conceptual framework and findings with a broad group of stakeholders and experts for further validation |
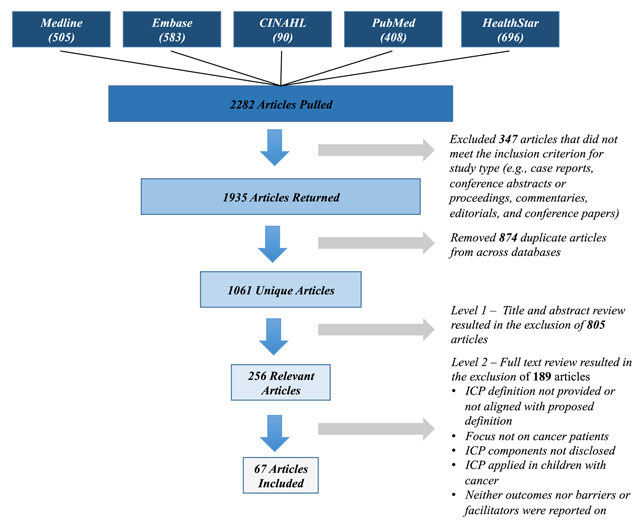
Figure 1
Overview of article retrieval, screening and data extraction stages.
Table 2
Descriptive features of included articles.
| Descriptive characteristics | Total (n = 67) | Relevant articles |
|---|---|---|
| Type | ||
| Surgical | 28 (41.8%) | [34363738394041424344454647484950515253545556575859606162] |
| Survivorship | 24 (35.8%) | [323563646566676869707172737475767778798081828384] |
| Palliative | 9 (13.4%) | [858687888990919293] |
| Comprehensive | 5 (7.5%) | [9495969798] |
| Systemic | 1 (1.5%) | [99] |
| Disease sites | ||
| Breast | 18 (26.9%) | [354563656667686972737779828384959698] |
| All | 11 (16.4%) | [7581858687888990919293] |
| Esophagus | 7 (10.4%) | [37474849505297] |
| Colorectal | 5 (7.5%) | [4143466480] |
| Multiple1 | 3 (4.4%) | [327678] |
| Prostate | 4 (6.0%) | [44586294] |
| Head and Neck | 4 (6.0%) | [34365461] |
| Gynecological2 | 5 (7.5%) | [5760707174] |
| Gastric, Bladder, Lung, Pancreatic, Brain, Larynx, and Testicular | 10 (14.9%) | [38394042515355565999] |
| Country | ||
| USA | 27 (40.3%) | [355051525354555657585960616273747576777879808182838493] |
| UK | 7 (10.4%) | [46474849729298] |
| Canada | 7 (10.4%) | [34363738656667] |
| Netherlands | 4 (6.0%) | [70718889] |
| Germany | 4 (6.0%) | [39406869] |
| Denmark, Italy, Australia, Singapore, Belgium, China, Japan, Spain, Sweden, Taiwan, Turkey and Multiple3 | 18 (26.9%) | [324142434445636485868790919495969799] |
| Medium | ||
| Paper | 21 (31.3%) | [383947495557596061626571747578808283859899] |
| Combination | 10 (14.9%) | [34356466707376777981] |
| Electronic | 6 (9.0%) | [324452639395] |
| Unclear | 30 (44.8%) | [363740414243454648505153545658676869728486878889909192949697] |
| Study design | ||
| Prospective observational (no control) | 25 (37.3%) | [35415052535658626364667273757677787980849596979899] |
| Pre and post comparison (with control) | 21 (31.3%) | [34363738394243444547484951555760618688919394] |
| Prospective observational (with control) | 13 (19.4%) | [46505354596981828589909295] |
| Randomized control trial | 8 (11.9%) | [4065676870717487] |
[i] 1Includes two or more disease sites;
2Includes ovarian, cervical, vaginal and/or endometrial cancer;
3The integrated care plan was implemented in multiple countries simultaneously.
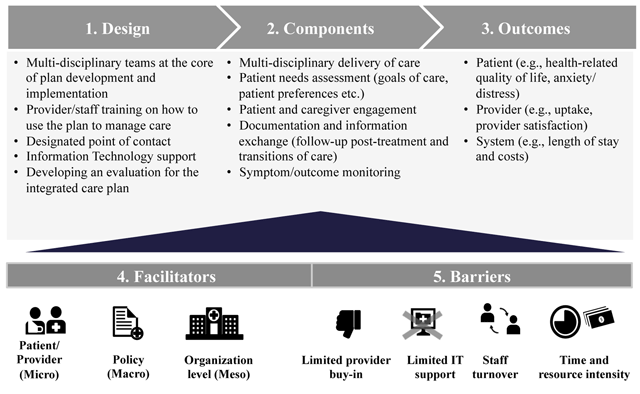
Figure 2
Integrated care planning for cancer care framework.
Table 3
Overview of measurement tools used to assess the impact of Integrated Care Plans.
| Indicators | Measurement tool/instrument |
|---|---|
| PATIENT | |
| Quality of life | • Short Form 36 Questionnaire [45] |
| • Short Form 12 [46] | |
| • European Organization for Research and treatment of Cancer Quality-of-life questionnaire [4664] | |
| Patient satisfaction | • Medical Outcomes Study – Patient Satisfaction Questionnaire [65] |
| • System Usability Scale (modified) [80] | |
| Anxiety/distress | • Spielberger State-Trait Anxiety Inventory [45] |
| • Brief Symptom Inventory [64] | |
| • Cancer Survivors Unmet Needs Scale [64] | |
| • Impact of Events Scale [65] | |
| • Profile of Mood States [65] | |
| • Distress Thermometer [71] | |
| • Patient-Perceived Coordination Index [94] | |
| • Hospital Anxiety and Depression Scale [72] | |
| Caregiver-reported outcomes | • Toolkit After-Death Family Member Interview [86] |
| • Views of Informal Carers Evaluation of Service Survey [86,88) | |
| • Evaluating Care and Health Outcomes for the Dying [92] | |
| • Family Satisfaction Survey [93] | |
| PROVIDER | |
| Uptake | • Chart reviews/retrospective audit [93] |
| Workflow – Time to complete care plan | • Provider self-report [80] |
| Provider satisfaction | • Telephone interviews [8693] |
| • System Usability Scale (modified) [80] | |
| • Consumer Assessment of Healthcare Providers and Systems Adult Specialty Care Clinician Questionnaire (modified) [80] | |
| SYSTEM* | |
| Length of stay | • Number of nights spent in the hospital after surgery |
| Post-operative complications | • Post-operative complication rates |
| Mortality | • In-hospital mortality |
| Readmissions | • Hospital readmissions |
| Costs | • Total costs of hospital stay |
| • Total cost of delivering the plan | |
| • Cost-effectiveness (i.e., quality adjusted life years gained for cost incurred) |
[i] *Since most system-level indicators represent standardized metrics individual references are not provided.
