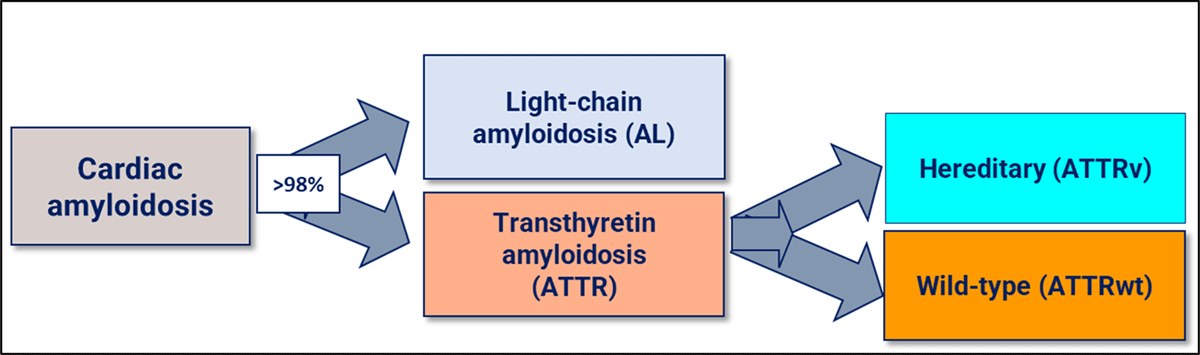
Figure 1
The most frequent amyloidosis subtypes that affect the heart.
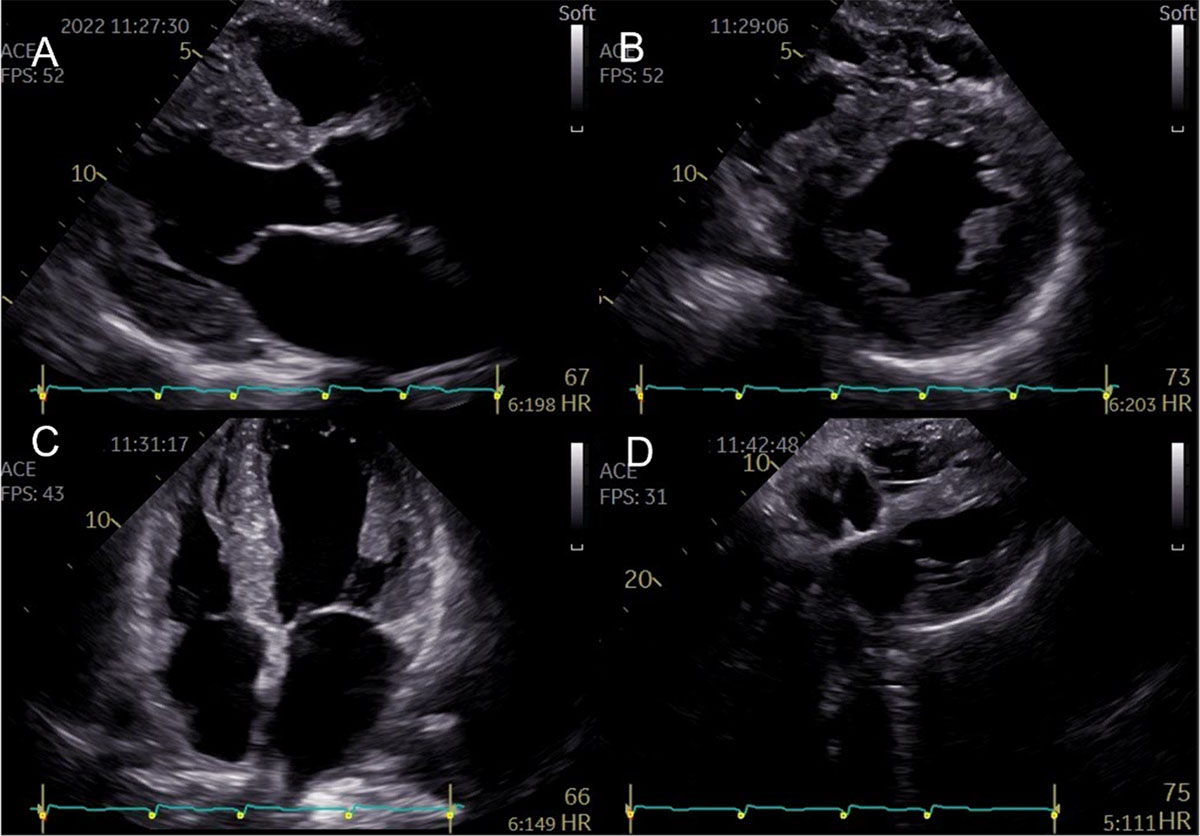
Figure 2
Representative two-dimensional echocardiographic findings of cardiac amyloidosis in a patient with ATTR-CM. (A) Parasternal longitudinal view (B) Short axis view (C) Apical 4-chamber view (D) Subcostal view Concentric left ventricular and right ventricle free wall hypertrophy, thickened interatrial septum, and atrioventricular valves. (Images courtesy of Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal).
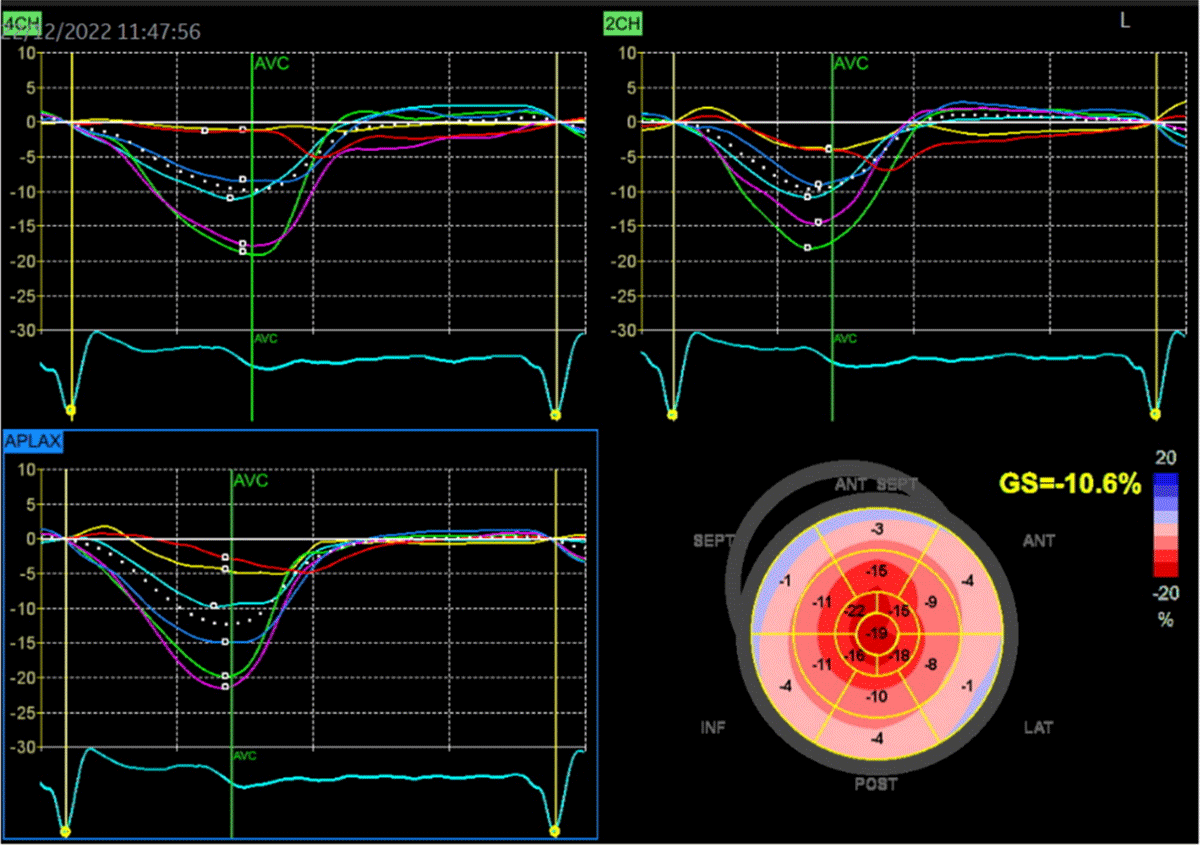
Figure 3
Two-dimensional speckle-tracking strain imaging echocardiography of a patient with ATTRwt-CM (the same patient as in Figure 2). Reduced left ventricular longitudinal strain in the middle and basal segments with relatively preserved strain in the apex (i.e., apical sparing) is observed. A bull’s eye is shown in the lower right panel (Images courtesy of Centro Hospitalar Universitário Lisboa Norte, Lisboa, Portugal).

Video 1
37-second recording in real-time showing typical echocardiographic features of a patient with ATTR-CM (the same patient as in Figures 2 and 3): concentric left ventricular hypertrophy, interatrial septum thickening, biatrial enlargement, and decreased global longitudinal strain with relative apical sparing. There is mild mitral and aortic regurgitation. The ejection fraction of both the left (LV) and right ventricle (RV) is preserved (4D analysis, LV in red, RV in blue). The patient gave informed consent for imaging and video use. https://dulcebritocardiologista.com/wp-content/uploads/2023/01/Amyloidosis-Final.edited.video_.mp4
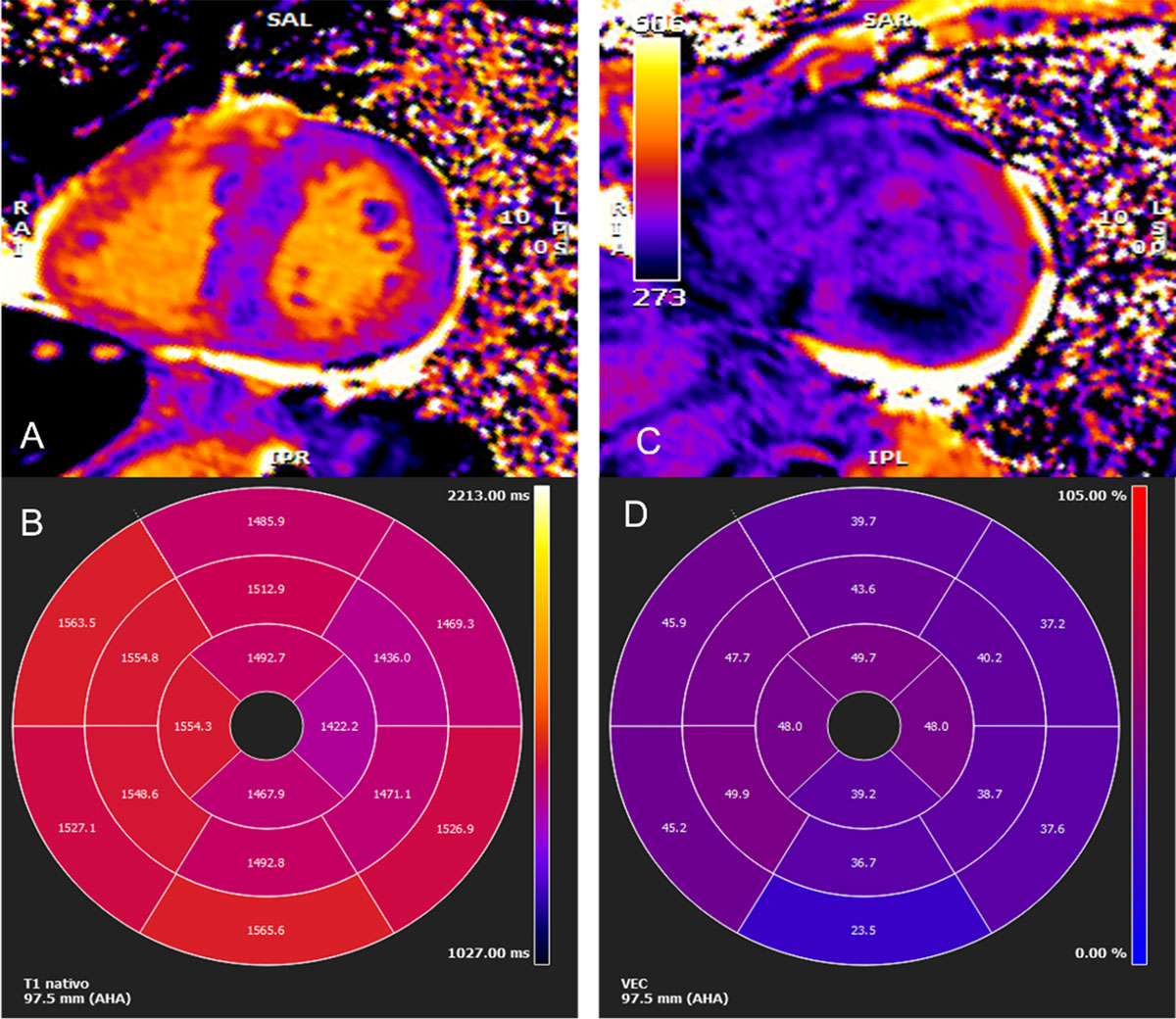
Figure 4
Cardiac magnetic resonance imaging. (A) Myocardial native T1 mapping (short axis) (B) Native T1 global polar map with abnormally increased T1 relaxation time (myocardial T1 values: 1,512±72 ms) (C) Post-contrast T1 (D) Global extracellular volume map, calculated from both native and post-contrast T1 myocardial values: in this case high above normal: 44±9%. (Images courtesy of Lusíadas Hospital, Lisboa, Portugal).
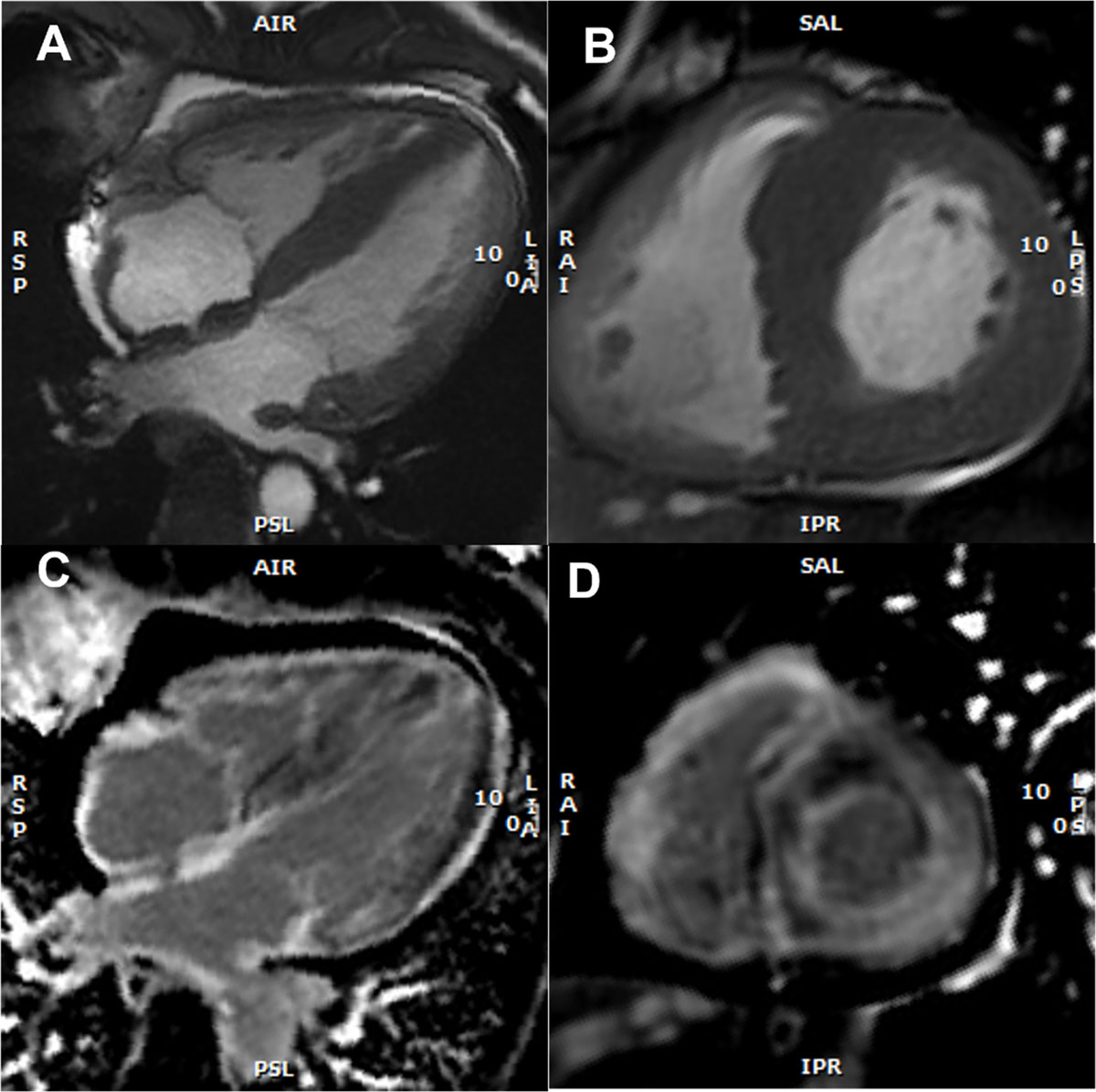
Figure 5
Cardiac magnetic resonance imaging findings with representative examples. (A) Cine: four-chamber view – SSFP (steady-state free precession) acquisition, depicting asymmetric left ventricular (LV) hypertrophy, inter-atrial septum thickening, and mild pericardial effusion (B) Short axis view – SSFP, asymmetric LV hypertrophy (C) Late gadolinium enhancement (LGE) at the 4-chamber view with subendocardial LGE at the left ventricle, atrial Wall, and inter-atrial septum (D) Short axis view, showing subendocardial and subepicardial LGE at the left ventricle and also at the right ventricle. (Images courtesy of Lusíadas Hospital, Lisboa, Portugal).
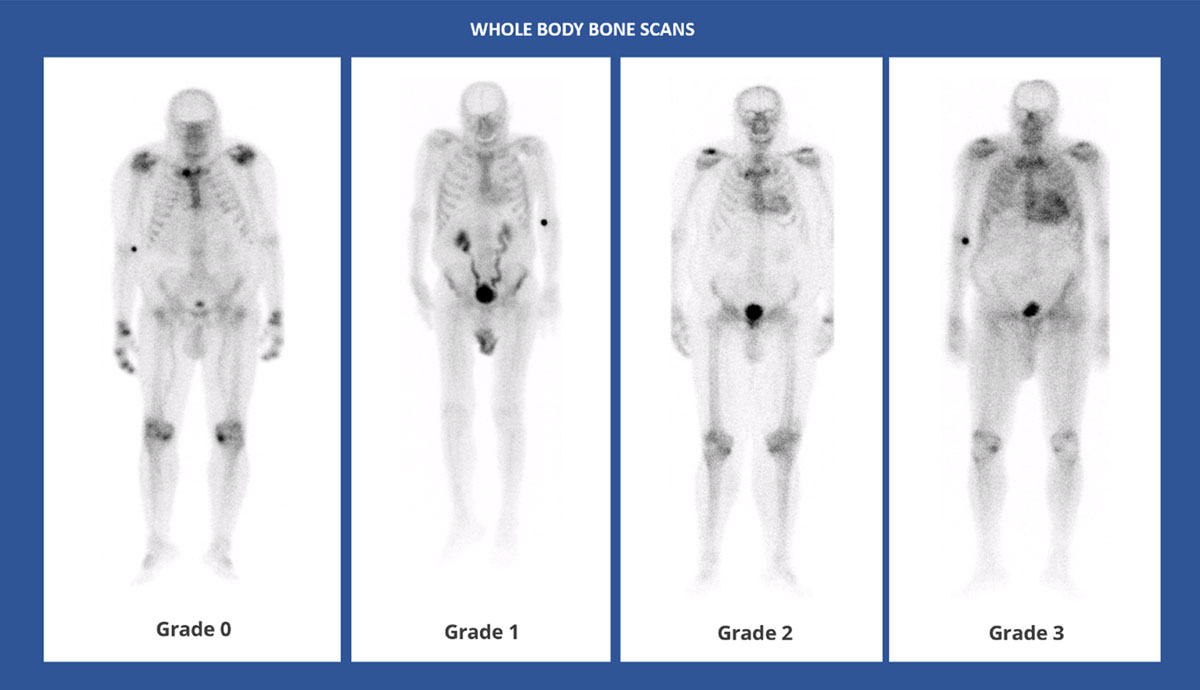
Figure 6
Whole body anterior planar views performed 3 hours post-injection of Tc99 m-DPD. Four different patients with different Perugini visual scores. (Images courtesy of Lisbon Medical School, Faculdade de Medicina da Universidade de Lisboa, Portugal).
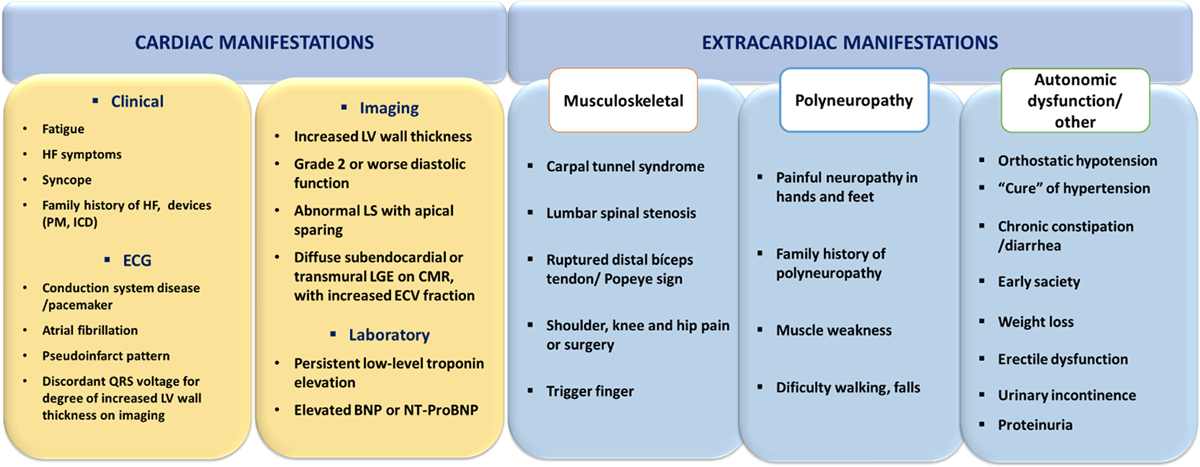
Figure 7
Clues (‘red flags’) to the presence of cardiac ATTR, Transthyretin amyloidosis; BNP or NT-proBNP, brain natriuretic peptides; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ECV, extracellular volume; HF, heart failure; ICD, implanted cardioverter defibrillator; LGE, late gadolinium enhancement; LS, longitudinal strain; LV, left ventricle; PM, pacemaker.
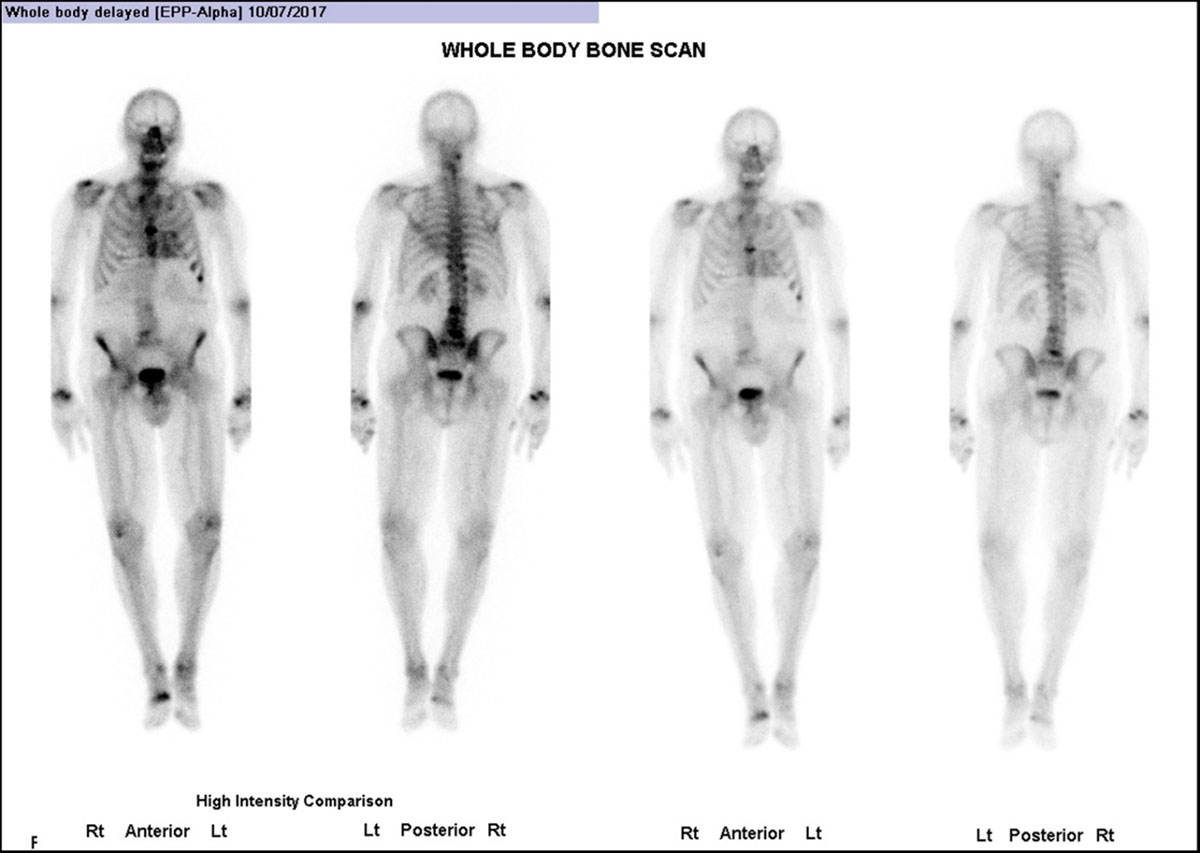
Figure 8
Whole body anterior and posterior planar views (shown at two intensities) performed 3 hours post-injection of Tc99 m HDP, investigating an 85-year-old man with back pain. He had no history of Heart Failure. (Images courtesy of Cabrini Health, Victoria, Australia).
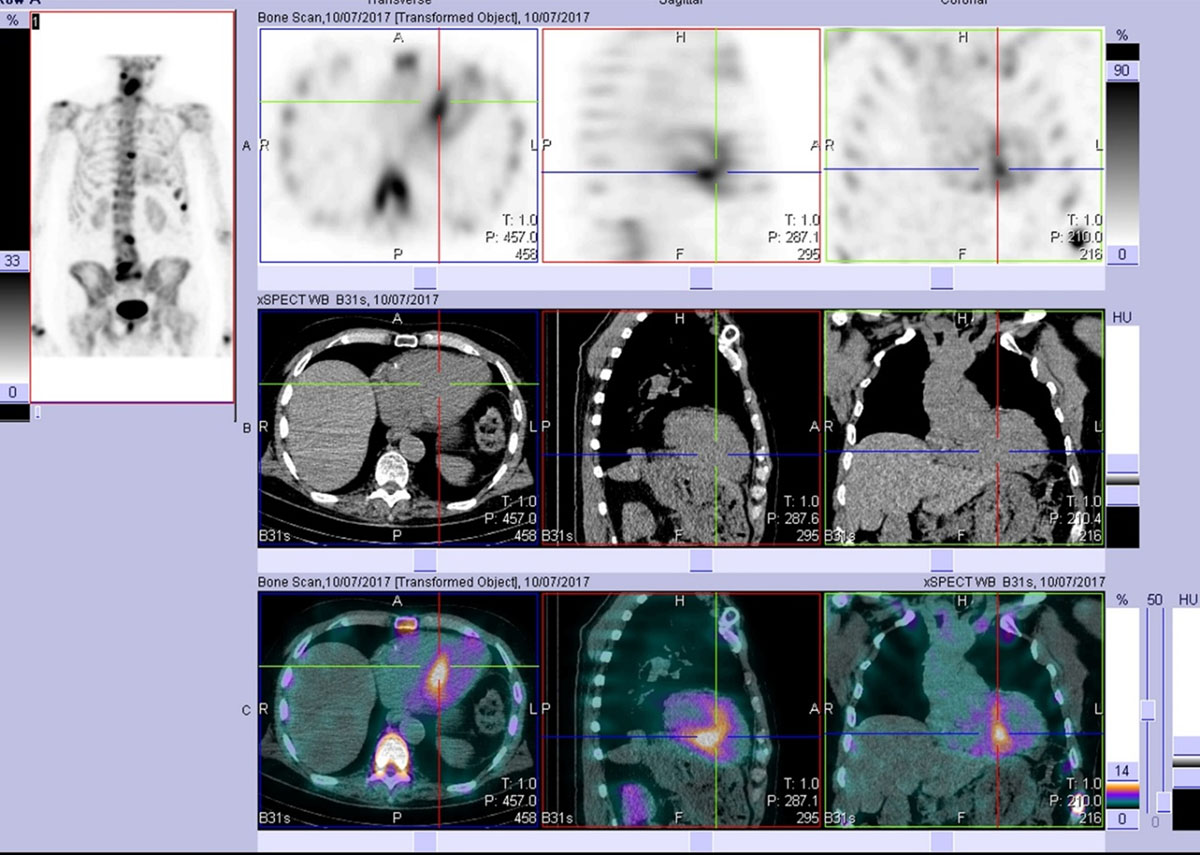
Figure 9
After viewing the planar study, a SPECT-CT was performed. This representative slice in axial, sagittal, and coronal plains (top row = SPECT, middle row = CT, bottom row = merged) confirms the uptake as cardiac and not blood pool. Echocardiogram at the time was normal. The patient developed echo and clinical features of cardiac ATTR amyloid approximately two years later. (Images courtesy of Cabrini Health, Victoria, Australia).

Figure 10
(A) ECG of a 48-year-old female (African ancestry), showing complete atrial-ventricular block on hospital admission after a syncope. She had mild hypertension, a history of paroxysmal atrial fibrillation, and right carpal tunnel syndrome. No known family history of amyloidosis. (B) ECG after pacemaker implantation. Echocardiographic images: (C) Parasternal longitudinal long-axis (D) Apical 3-chamber views, showing only mild and localized (basal septum) LVH (12 mm). Cardiac magnetic resonance (E-H) was also normal: no cardiac hypertrophy, and (F, H) no late gadolinium enhancement; (I) 99 mTc-3,3-diphosphono-1,2 propanodicarboxylic acid (DPD) scintigraphy (chest images) showed no cardiac uptake three hours after radiotracer administration. Genetic testing identified the pathogenic mutation p.Val142Ile in the TTR gene (no other mutations in a large panel of genes studied by next-generation sequencing). Of her three children (adolescents), two have the mutation (no phenotype). (Images courtesy of Centro Hospitalar Universitário Lisboa Norte, Lisbon, Portugal).
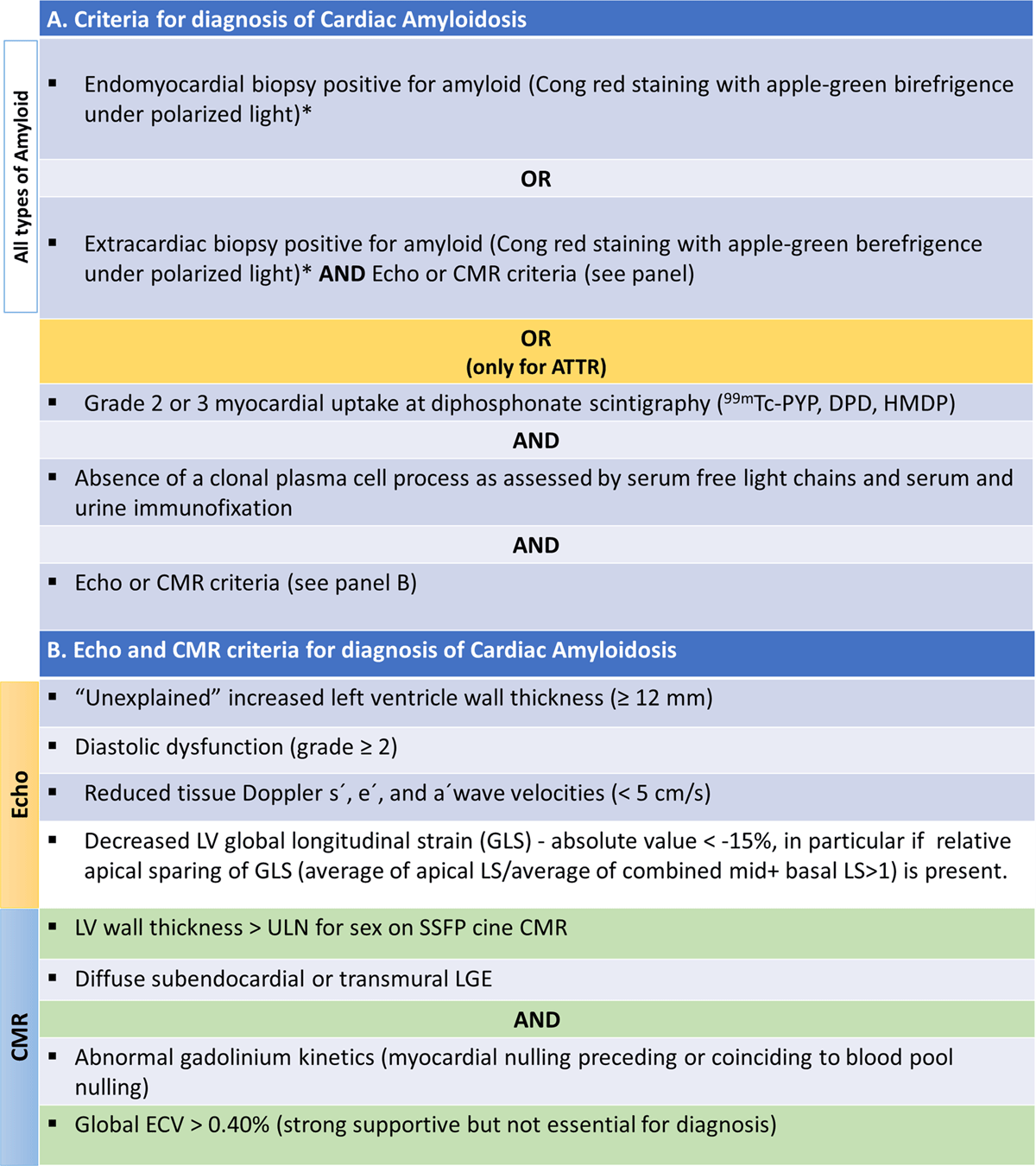
Figure 11
Diagnosis criteria of cardiac amyloidosis. (A) Criteria for diagnosis of cardiac amyloidosis. (B) Echocardiographic and cardiac magnetic resonance criteria for diagnosis of cardiac amyloidosis. Modified from [22104].
*Classification of the amyloid fibril protein must follow (mass spectrometry or immunohistochemistry). ATTR, Transthyretin Amyloidosis; CMR, Cardiac magnetic resonance; Echo, echocardiogram; ECV, extracellular volume; LS, longitudinal strain; LGE, late gadolinium enhancement; LV, left ventricle; SSFP, steady-state free precession; ULN, upper limit of normal.
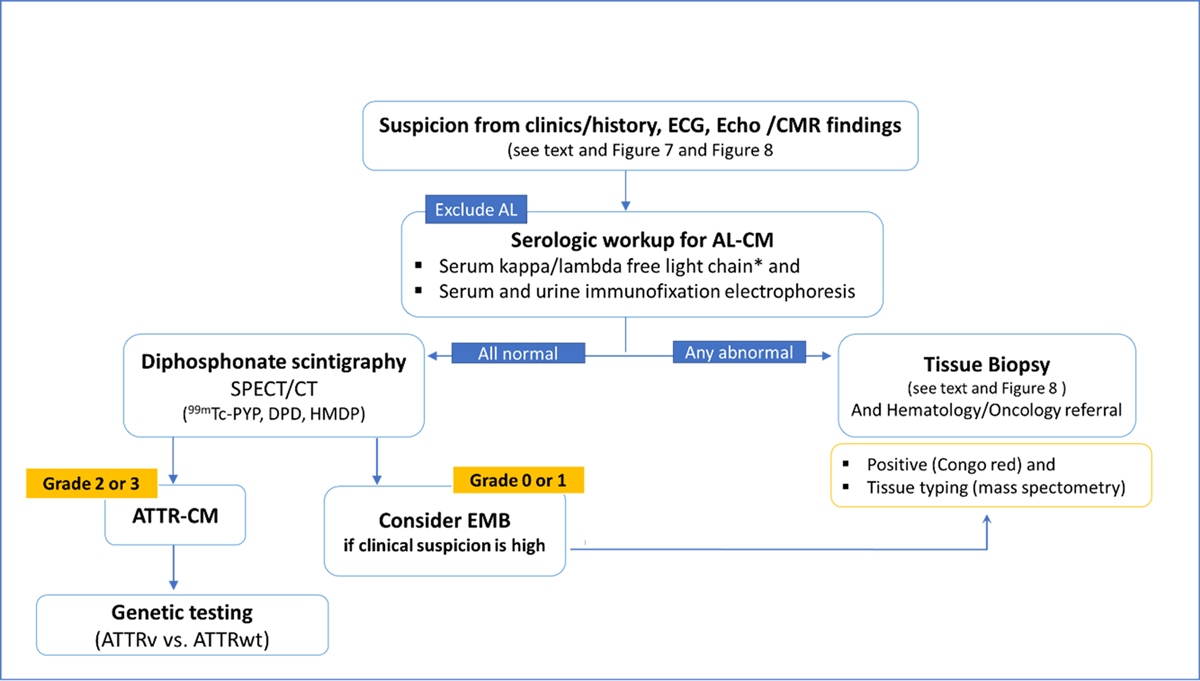
Figure 12
Algorithm for the diagnosis of Cardiac Amyloidosis.
*Reference intervals for free light chain ratio according to renal function must be considered; AL-CM, Light chain cardiac amyloidosis; ATTR-CM, Transthyretin cardiac amyloidosis; ATTRv, genetic (hereditary) ATTR-CM; ATTRwt, wild type ATTR-CM; CMR, cardiac magnetic resonance; DPD, 3,3-diphosphono-1,2-propanodicarboxylic acid; Echo, echocardiography; EMB, endomyocardial biopsy; HMDP, hydroxymethylene-diphosphonate; K/L, kappa/lambda; PYP, pyrophosphate; 99 mTc, technetium-99 m.
Table 1
Potential consequences arising from a delayed diagnosis or a misdiagnosis.
| CONSEQUENCES OF DELAYED DIAGNOSIS OR MISDIAGNOSIS: SUMMARY KEY POINTS |
|---|
|
|
|
Table 2
Treatment of Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM).
| TREATMENT OF ATTR-CM: SUMMARY KEY POINTS |
|---|
|
| Heart failure |
| Arrhythmias |
| Thromboembolism |
| Orthostatic hypotension |
| Correction of aortic stenosis and other valvular defects (if indicated) |
| Heart transplantation/other measures for advanced heart failure |
|
| Transthyretin stabilizers |
| Transthyretin silencers |
| Other (possible non-genetic and genetic therapies) |
|
| Exercise & Nutrition |
| Vaccines |
| Individualized approach to physical and emotional patient’s needs |
Table 3
The patient’s perspective: what’s more important.
| THE PATIENT’S PERSPECTIVE: SUMMARY KEY POINTS |
|---|
|
| To have an early and accurate diagnosis! |
| What’s necessary? To increase awareness of the disease |
| Public campaigns (directed to the general population) Sensibilization of media Collaborative work: Amyloidosis support groups for patients, National Cardiology Societies, industry (sponsors) |
| Disseminate the diagnostic algorithms & Consensus documents and existing recommendations |
|
| To have the facility of genetic testing |
| To get from his/her doctor full information about the disease (patients & family, and caregivers) |
| To be followed in centres with experience in managing amyloidosis/Excellence centres (Ecs)* |
| Straight relationship between Ecs and the physician’s doctor in the community |
| To get full information about treatment and prognosis, and discussion with the patient about its expectations |
[i] * Should include genetic counseling in case of hereditary transthyretin amyloidosis.

Video 2
Testimonials of patients with amyloidosis. https://dulcebritocardiologista.com/wp-content/uploads/2023/01/Amyloidosis-The-Voice-of-the-Patient.mp4
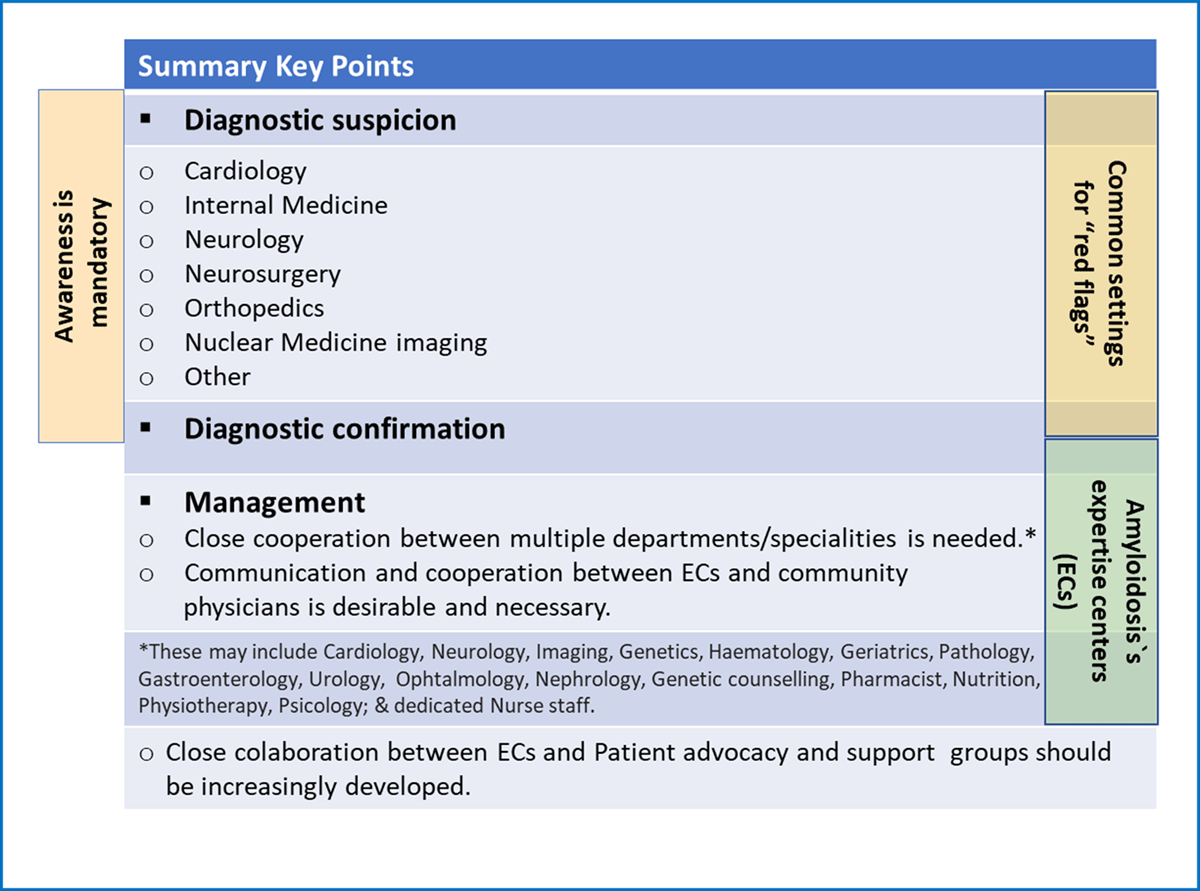
Figure 13
Transthyretin Amyloidosis: a multidisciplinary approach.
