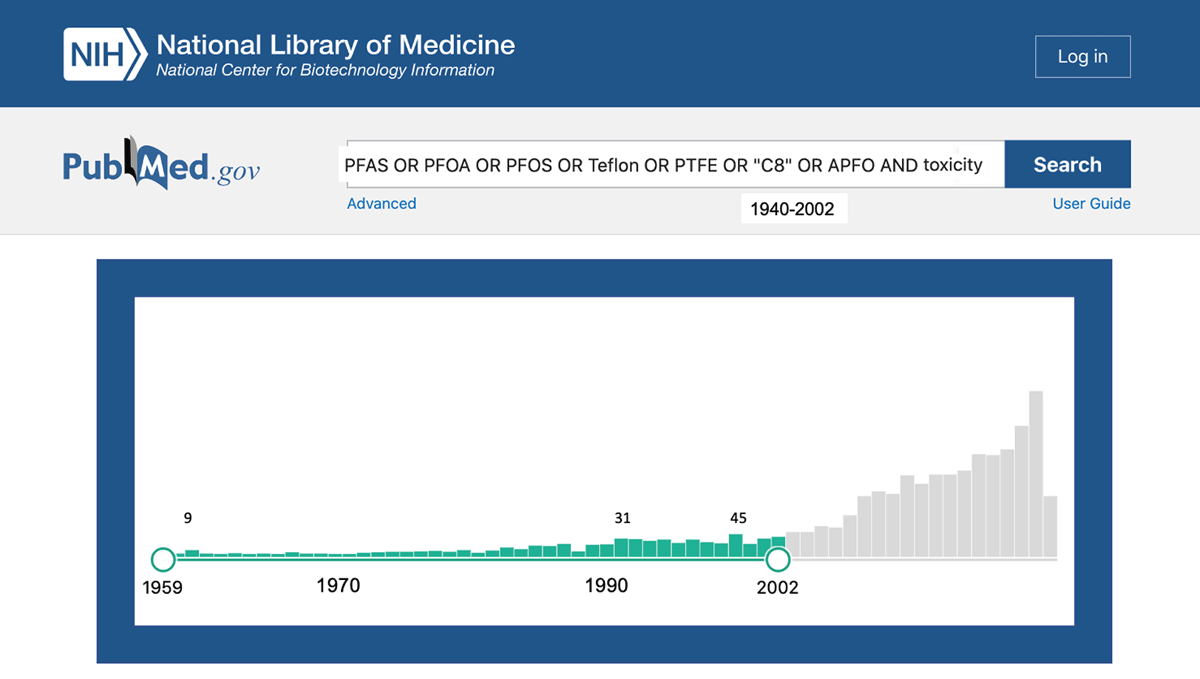
Figure 1
Hits from PubMed literature search of “PFAS OR PFOA OR PFOS OR Teflon OR PTFE OR “C8” OR APFO AND toxicity” from 1940–2021 (March), highlighting date range of study, 1959, the earliest hit, to 2002, the year of the Leach vs. DuPont filing.
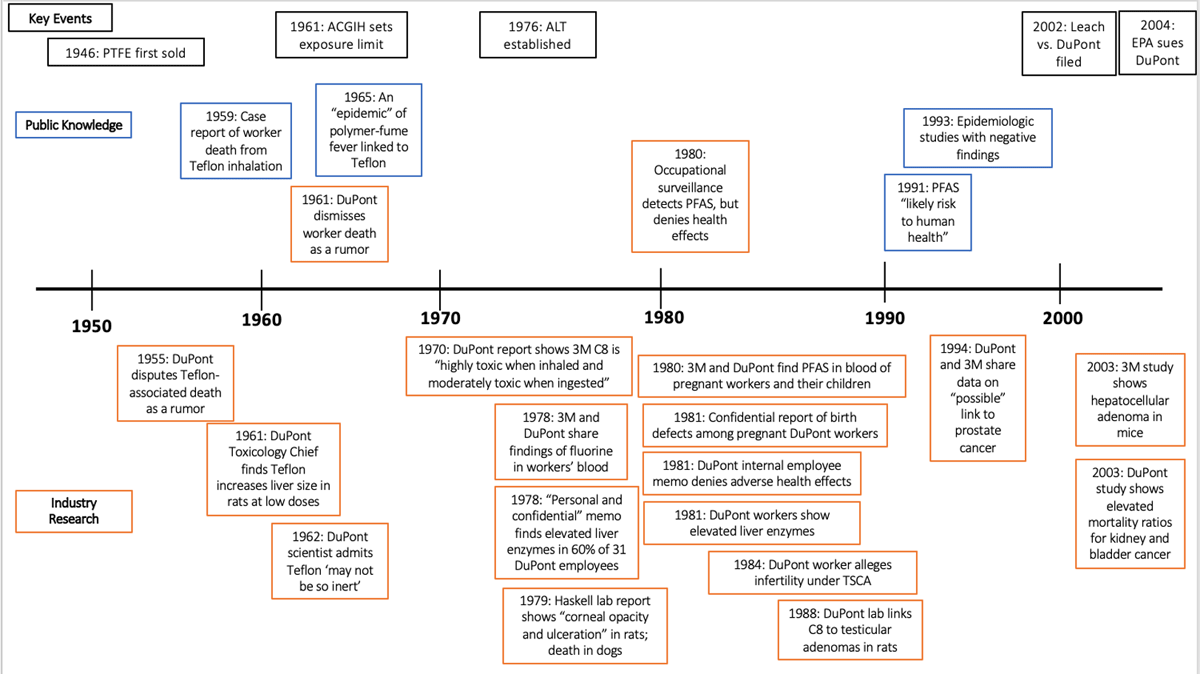
Figure 2
Timeline of notable public health research and health-related industry findings on PFAS health effects, with key historical events in black. The period of interest extends from 1940 to the 2002 Leach vs. DuPont filing, though we include two papers submitted by industry to the EPA after that filing with positive findings of harm and the 2004 lawsuit the EPA subsequently filed, for context. Industry research is in orange; non-industry papers are in blue. Above the timeline are papers in the public domain, and below are papers in the private domain.
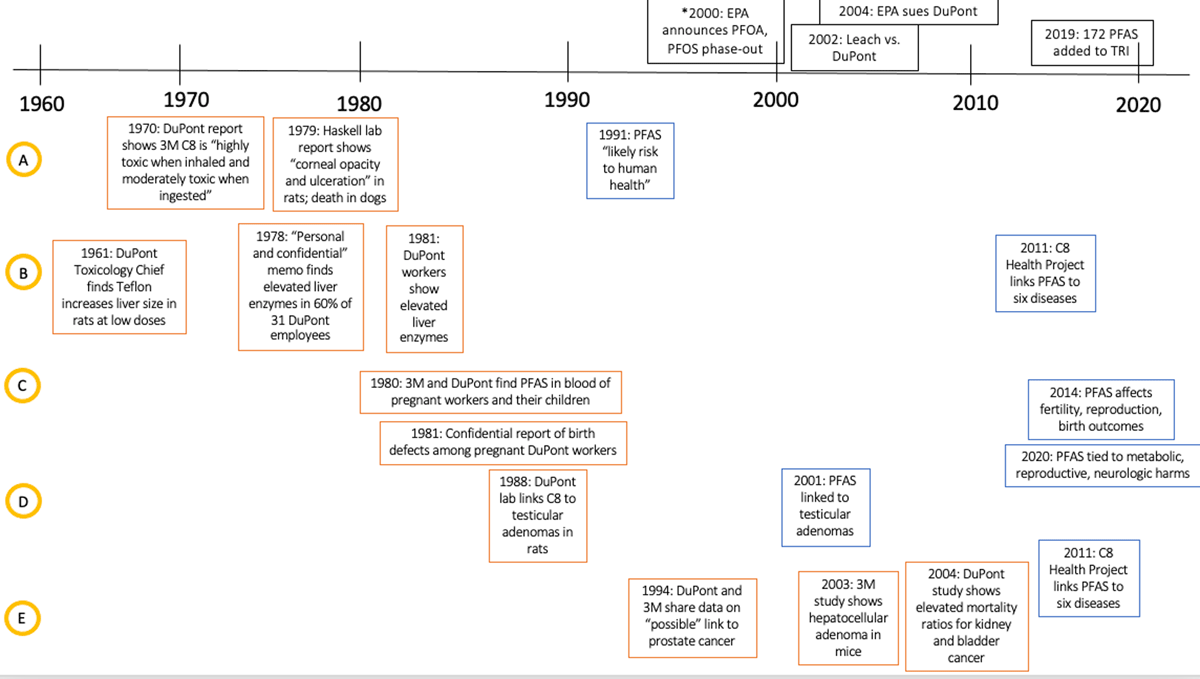
Figure 3
An extended timeline following five health outcomes of interest, showing when information was known by industry (in orange) and in the public domain (in blue). A = Systemic toxicity, B = Liver toxicity, C = Reproductive outcomes, D = testicular adenomas, E = Other cancer risk.
Table 1
Evidence of Industry Influence on Public Understanding of PFAS toxicity (strategies adapted from White and Bero 2010).
| INDUSTRY STRATEGY | YEAR | INDUSTRY DOCUMENTS EVIDENCE |
|---|---|---|
| Influence Research Question: Industry decides what to study, or not, in order to produce evidence detracting from harms of their product. | ||
| 1978 | DuPont’s occupational physician noted “unusually high” liver enzyme elevations but dismissed findings as clinically insignificant, despite inadequate statistical power, neglecting to pursue research [87]. | |
| 1981 | DuPont’s contract lab used alternate protocol to run liver enzyme samples of exposed employees; after “reevaluation” the majority of concerning tests were ruled “normal [88].” | |
| Fund and Publish Favorable Research: Industry funds and publishes research that concludes their products were safe. | ||
| 1996 | 3M funded a study of occupationally exposed men and found no clinical hepatic toxicity [79]. | |
| Suppress Unfavorable Research: Industry documents harms that are not made public. | ||
| 1961 | C6, C9, and ART increased liver size of rats even at low doses, should be handled “with extreme care [89].” | |
| 1970 | Industry Lab report finds C8 “highly toxic when inhaled and moderately toxic when injected [90].” | |
| 1979 | DuPont’s Haskell labs found “corneal opacity and ulceration” in rats, death in two dogs from ingesting APFO in low doses [91]. | |
| 1981 | Record of two children born to exposed workers with eye and facial defects; PFAS found in cord blood in a third [92]. | |
| 1981 | Confirmed fetal eye changes related to C8[93]. | |
| 1990 | DuPont lab links C8 to testicular adenomas in rats [94]. | |
| 1994 | 3M knew of “possible” prostate cancer and shared with DuPont [95]. | |
| Distort Public Discourse: Industry works to distort public discourse, both within and outside the companies. | ||
| 1980 | 3M internal communications says that C8 is “about as toxic as table salt [96].” | |
| 1981 | DuPont and 3M joint employee communications denies workers have been exposed at levels that could cause adverse health effects, denies adverse pregnancy outcomes [97]. | |
| 1991 | DuPont public press release denies adverse health effects [98]. | |
| 2000 | With Tenant lawsuit on the horizon, email from DuPont manager says, “the plant recognizes it must get public first… better late than never [99].” | |
| 2006 | DuPont demands the EPA certify Teflon as safe and deny any adverse health effects linked to PFOA [100]. | |
| Change or Set Scientific Standards: Industry sets occupational safety standards within the workplace as well as public safety standards. | ||
| 1991 | DuPont insisted no EPA notification was warranted, years after determining PFAS were a chronic hazard [101]. | |
| 2000 | Public water utility informs customers that DuPont insists its own exposure guidelines are health protective [102]. | |
| Targeted Dissemination: Industry strategically disseminates information to key policymakers. | ||
| N/A | ||
