Table 1
Characteristics of participants stratified by age-classes <65 or ≥65 years old.
| ADMITTED PATIENTS | P-VALUE | ||||
|---|---|---|---|---|---|
| TOTAL N. 206 (100%) | AGED < 65 N. 151 (100%) | AGED ≥ 65 N. 55 (100%) | |||
| Sex | M | 124 (60) | 91 (74) | 33 (26) | 0.9 |
| F | 82 (40) | 60 (75) | 22 (25) | ||
| Age Median (IQR) | 39 (16–92) | 32 (16–63) | 71 (65–92) | 0.00 | |
| Nationality | Italian | 101 (49) | 54 (53) | 47 (47) | 0.01 |
| Non-Italian | 105 (51) | 97 (94) | 8 (6) | ||
| Homeless | 17 (8) | 17 (100) | 0 (0) | NA | |
| HIV + status | 6 (3) | 5 (84) | 1 (16) | 0.9 | |
| Previous contact with tb patient | 60 (30) | 52 (88) | 8 (12) | 0.00 | |
| Hospital stay, median | 44,5 | 43 (7–230) | 24 (4–45) | 0.02 | |
| Diagnostic delay, median | 76 | 127 (2–450) | 43 (5–120) | 0.01 | |
| Type | Pulmonary TB | 135 (65) | 107 (80) | 28 (20) | 0.00 |
| Extrapulmonary TB | 64 (31) | 38 (60) | 26 (40) | 0.01 | |
| Miliary TB | 7 (4) | 6 (86) | 1 (14) | 0.7 | |
| Respiratory symptoms | 124 (60) | 101 (81) | 25 (19) | 0.02 | |
| Type of diagnosis | Culture positive | 140 (68) | 107 (77) | 33 (23) | 0.03 |
| Radiological | 42 (20) | 31 (74) | 11 (26) | 0.03 | |
| NAT | 5 (2) | 2 (40) | 3 (60) | 0.2 | |
| Histological | 19 (9) | 10 (48) | 9 (52) | 1 | |
| IGRA test | 123 (62) | 97 (78) | 26 (22) | 0.08 | |
| Initial Therapeutic Scheme, n (%) | R+H+E+Z | 166 (80) | 123 (75) | 44 (25) | 0.9 |
| Drug regimen without Z Including Amikacin | 5 (2) | 2 (40) | 3 (60) | 0.6 | |
| Drug without Z regimen including fluoroquinolone | 1 (1) | 1(100) | 0 (0) | NA | |
| Resistance Pattern | Monoresistance | 34 (16) | 25 (73) | 9 (27) | 0.3 |
| H | 18 (9) | 14 (77) | 4 (23) | 0.7 | |
| R | 13 (6) | 7 (54) | 5 (46) | 0.5 | |
| Z | 3 (1) | 2 (66) | 1 (34) | 1 | |
| MDR | 9 (4) | 9 (100) | 0 (0) | 0.1 | |
| Adverse events and management, n (%) | Adverse events | 49 (25) | 36 (73) | 13 (27) | 0.3 |
| Therapeutic Shift | 24 (12) | 19 (80) | 6 (20) | 0.5 | |
| Outcomes | Exitus | 4 (1) | 3 (75) | 1 (25) | 0.8 |
| Successful treatment | 117 (57) | 71 (60) | 46 (40) | 0.1 | |
| Unsuccessful treatment | 65 (32) | 61 (94) | 4 (6) | 0.00 | |
| Treatment on going | 20 (10) | 16 (66) | 4 (34) | 0.1 | |
Table 2
Characteristics of adverse events in the 49 patients who reported them.
| CHARACTERISTICS | TOTAL N. 49 (100%) | |
|---|---|---|
| Type of Adverse events, n (%) | Hepatitis | 27 (55) |
| Neurological | 5 (11) | |
| Ocular damage/decrease in visual acuity | 5 (11) | |
| Itching/skin rash | 11 (22) | |
| Acute renal failure | 1 (1) | |
| Severity of Adverse events, n (%) | Mild | 25 (51) |
| Moderate | 18 (37) | |
| Severe | 6 (22) | |
| Adverse events management, n (%) | Therapeutic shift | 24 (49) |
| Temporary suspension of all treatment | 9 (18) | |
| Support therapy and no change of treatment | 16 (33) |
Table 3
Predictors of unsuccessful treatment for active pulmonary tuberculosis.
| CHARACTERISTICS | UNIVARIATE ANALYSIS O.R. | MULTIVARIATE ANALYSIS ADJ-O.R. |
|---|---|---|
| Age < 65 | 1.22 (0.98–1.64) | 3.91 (1.72–4.21)* |
| Female | 0.48 (0.16–0.90) | 0.68 (0.16–1.10) |
| Non-Italian nationality | 1.40 (1.28–1.76) | 4.45 (2.22–4.98)* |
| Homeless | 1.51 (1.28–2.03) | 3.23 (2.58–4.54)* |
| Diagnostic delay | 1.72 (1.08–2.01) | 2.55 (1.98–3.77)* |
| Length of hospitalisation | 1.15 (0.68–1.54) | 1.42 (0.85–1.87) |
| Pulmonary TB | 1.21 (0.28–1.23) | 1.13 (0.88–1.94) |
| Extrapulmonary TB | 1.14 (0.88–1.58) | 1.84 (0.91–2.78) |
| Respiratory symptoms | 1.26 (0.85–1. 72) | 1.23 (1.10–1.90)* |
| Culture positive | 1.64 (0.38–1.78) | 1.24 (0.38–1.48) |
| Monoresistance, n | 0.35 (0.12–0.60) | 0.75 (0.45–1.34) |
| MDR | 1.21 (0.89–1.73) | – |
| R + H + E + Z | 0.79 (0.68–1.21) | 1.10 (0.83–2.21) |
| Drug regimen without Z including Amikacin | 0.59 (0.48–1.21) | – |
[i] Legend
R: Rifampicin
H: Isoniazid
E: Ethambutol
Z: Piraldine
TB: Tuberculosis.
Table 4
Predictors of adverse events for active pulmonary tuberculosis.
| CHARACTERISTICS | UNIVARIATE ANALYSIS O.R. | MULTIVARIATE ANALYSIS ADJ-O.R. |
|---|---|---|
| Age <65 | 1.18 (0.81–3.47) | 1.73 (1.31–2.49)* |
| Female | 0.48 (0.26–1.09) | 0.38 (0.16–1.05) |
| Non-Italian nationality | 1.40 (0.88–1.76) | 0.95 (0.82–1.59) |
| Homeless | 0.75 (0.55–1.06) | 0.88 (0.55–1.18) |
| HIV status | 0.44 (0.28–1.06) | – |
| Diagnostic delay | 1.10 (0.68–1.41) | 1.45 (0.75–1.97) |
| Length of hospitalisation | 1.25 (0.88–1.71) | 1.82 (1.35–2.57)* |
| Pulmonary TB | 0.76 (0.38–1.08) | 1.15 (1.02–1.35)* |
| Extrapulmonary TB | 0.70 (0.48–1.16) | 0.83 (0.62–1.16) |
| Respiratory symptoms | 1.21 (0.88–1.76) | 0.95 (0.82–1.58) |
| Culture positive | 1.20 (0.78–1.56) | 1.35 (1.12–1.82)* |
| Monoresistance, n | 0.64 (0.48–0.96) | 0.95 (0.82–1.29) |
| MDR | 1.40 (0.88–1.76) | – |
| R + H + E + Z | 1.04 (0.88–1.46) | 0.85 (0.72–1.64) |
| Drug regimen without Z including Amikacin | 0.39 (0.28–0.56) | – |
[i] Legend
R: Rifampicin
H: Isoniazid
E: Ethambutol
Z: Piraldine
TB: Tuberculosis.
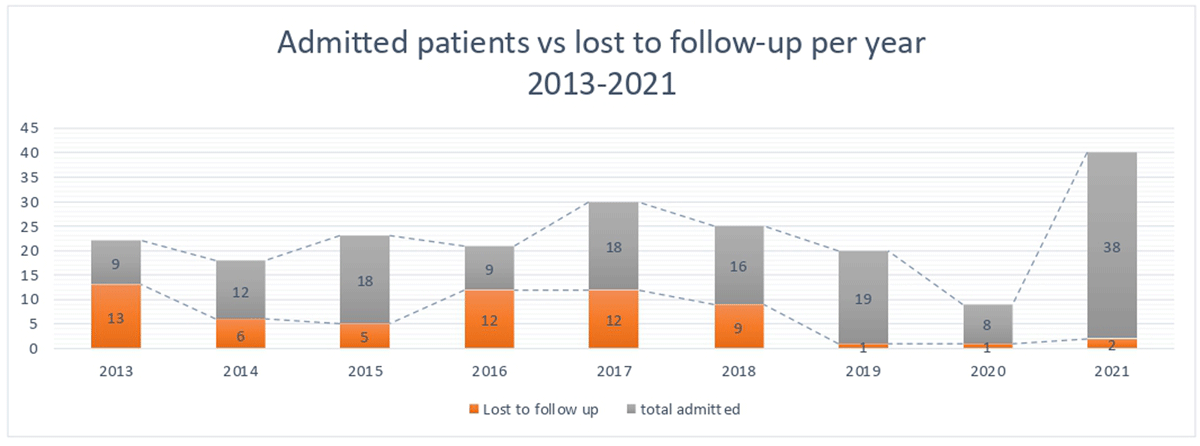
Figure 1
2013–2021 trend of admitted patients and lost to follow up.
