Fig. 1.
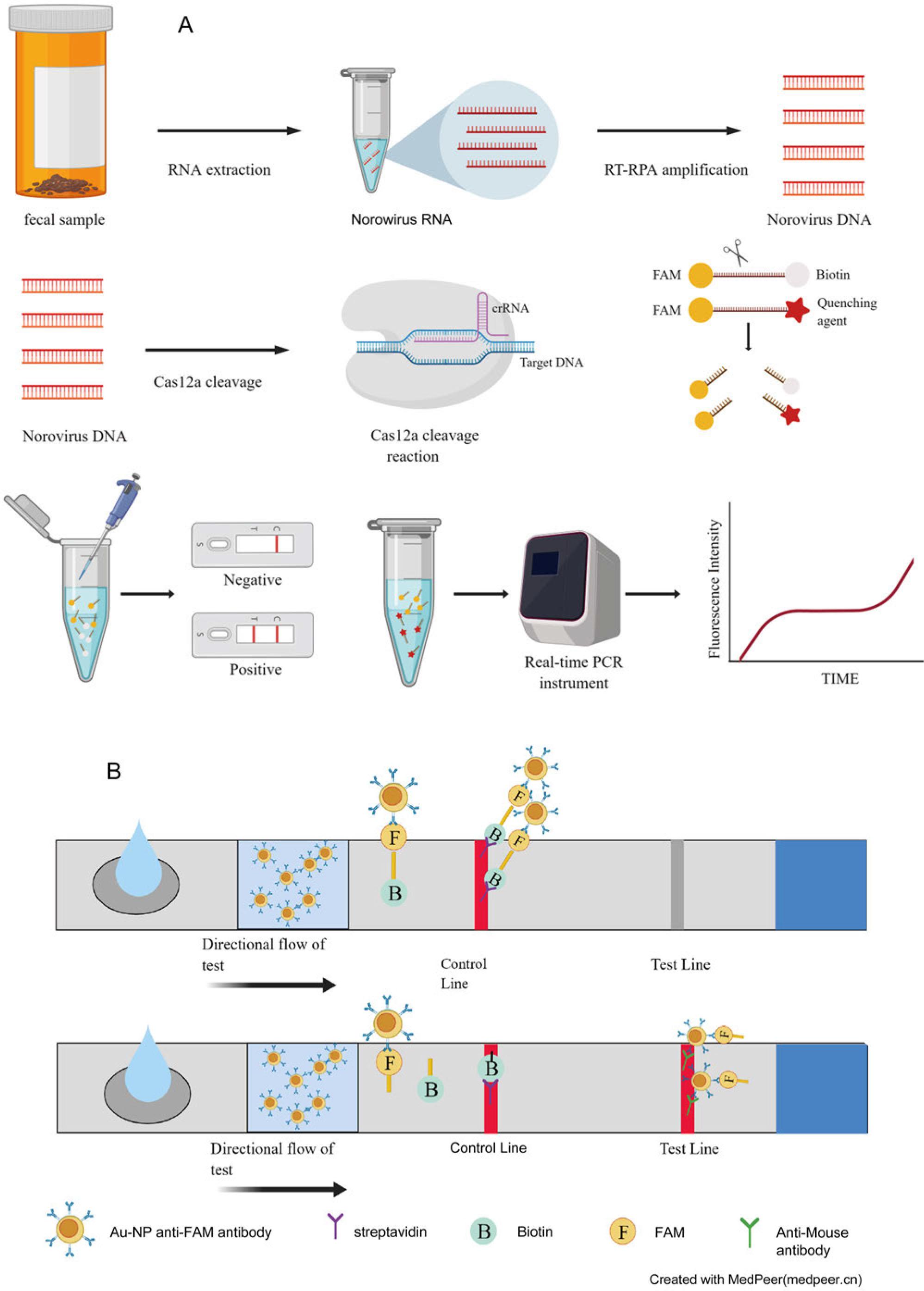
Fig. 2.
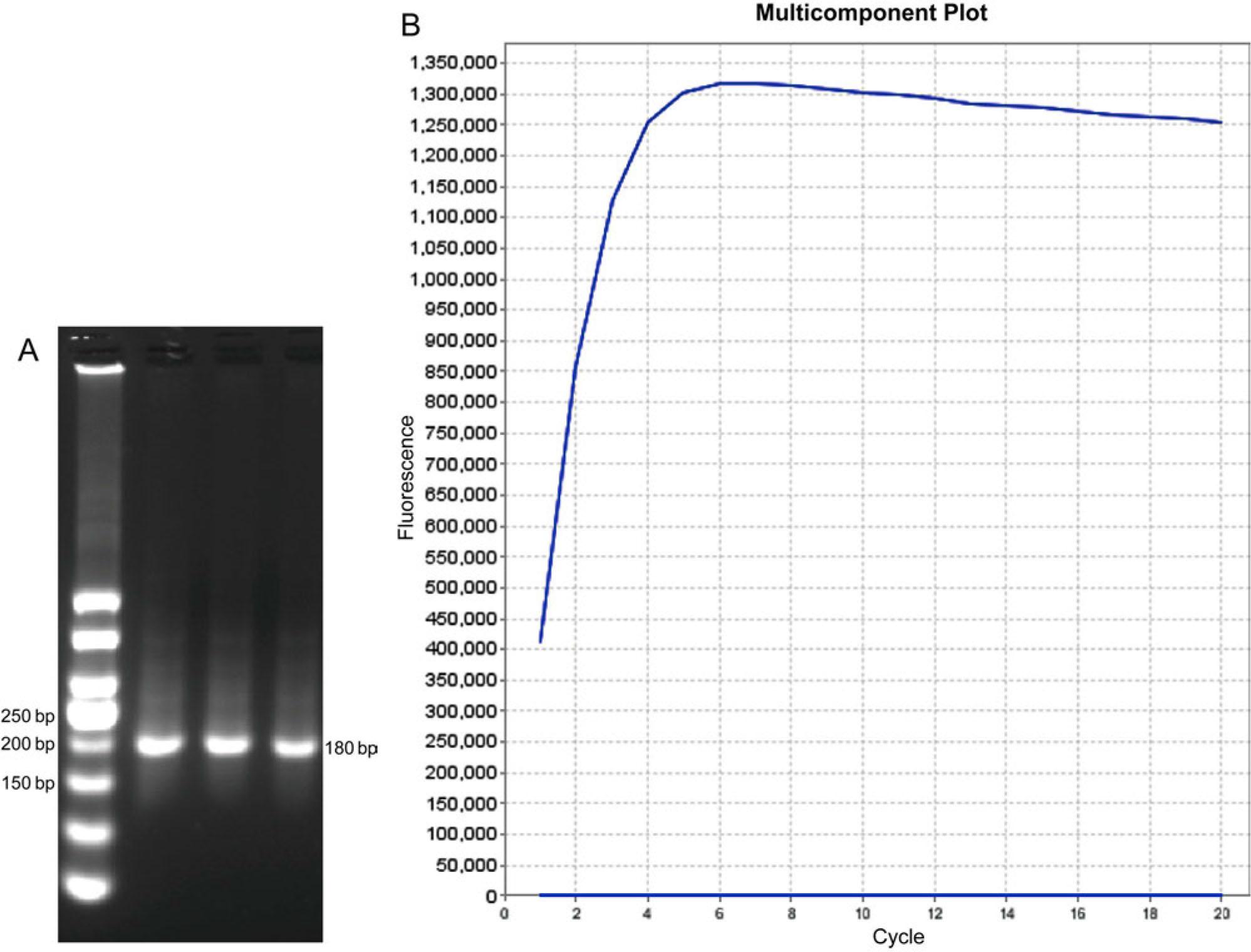
Fig. 3.
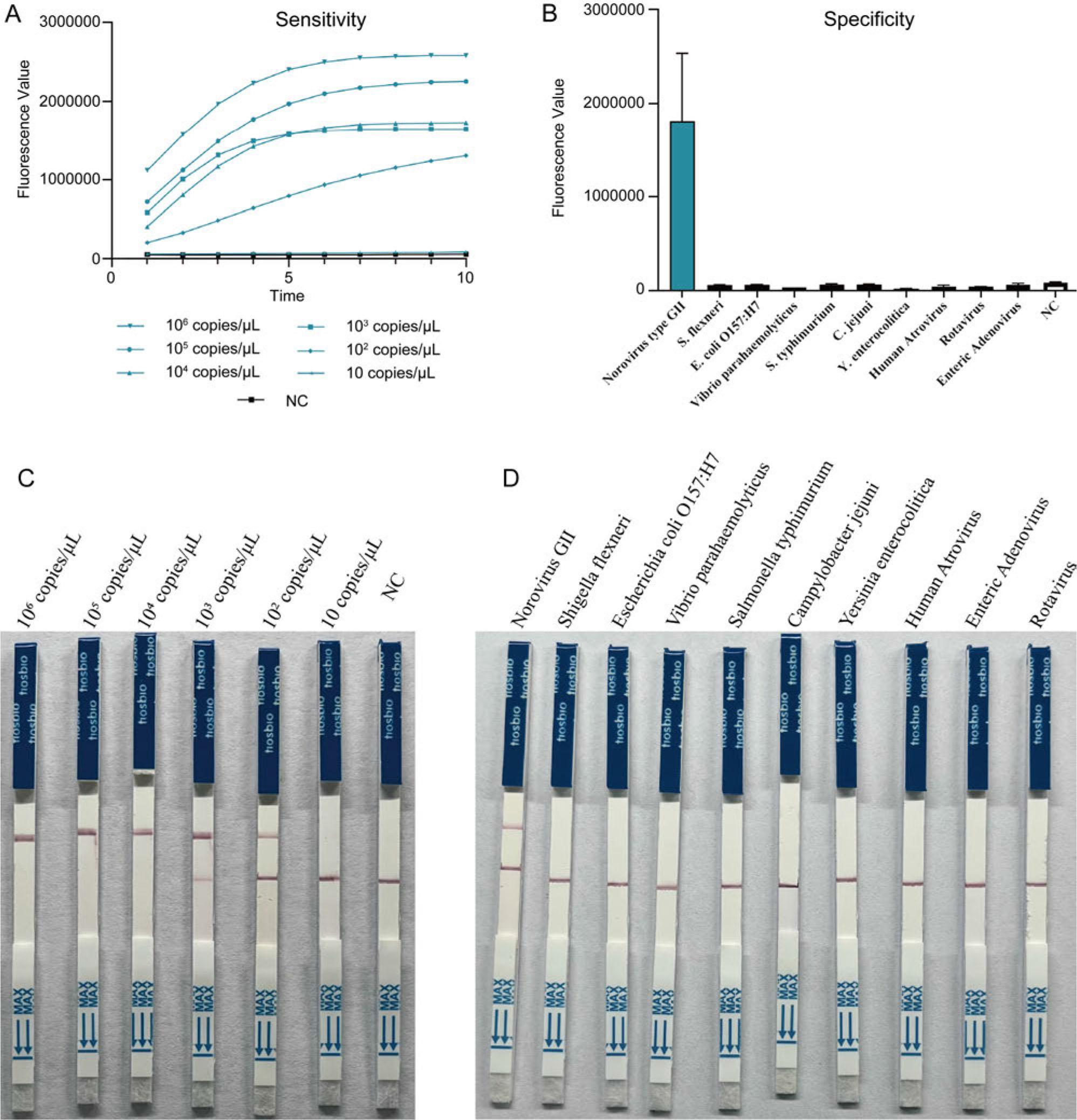
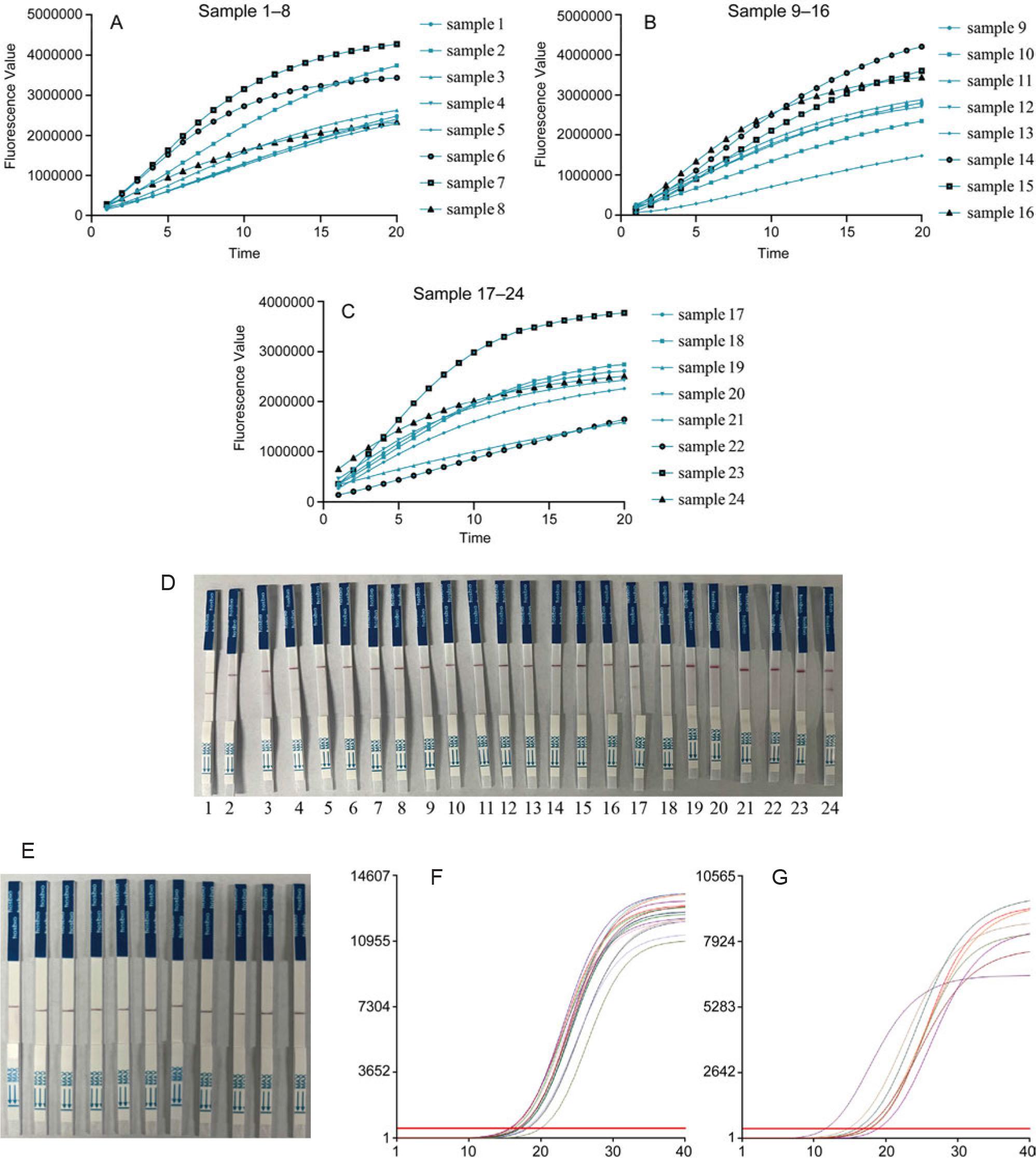
Fig. 4.
Comparison between RPA-cas12a method and qPCR_
| RPA-Cas12a | Quantitative Real-Time PCR | Total | |
|---|---|---|---|
| + | − | ||
| + | 24 | 0 | 24 |
| − | 0 | 11 | 11 |
| Total | 24 | 11 | 35 |
Bacteria and viruses involved in this study_
| Name | Source of strains |
|---|---|
| Shigella flexneri | CMCC(B)51572 |
| Escherichia coli O157:H7 | NCTC12900 |
| Vibrio parahaemolyticus | ATCC® 17802™ |
| Salmonella Typhimurium | Clinical isolates |
| Campylobacter jejuni | ATCC® 33291™ |
| Yersinia enterocolitica | CMCC(B)50024 |
| Human astrovirus | Clinical isolates |
| Rotavirus | ATCC® VR-2274™ |
| Enteric adenovirus | ATCC® VR-930™ |
Primers and crRNA sequences_
| Oligonucleotide | Sequence (5’–3’) |
|---|---|
| RPA forward primers | CCTCTCTTCACGGACCCTCTTTCTACAGC |
| RPA reverse primers | TTCATTCACAAAATTGGGAGCCAGATTGC |
| crRNA | AGUGCCUGGGAGAAAGAUGUCGUUU |
| ssDNA reporters | FAM-TTATTATT-BHQ1 |
| FAM-TTATTATT-Biotin |