Fig. 1
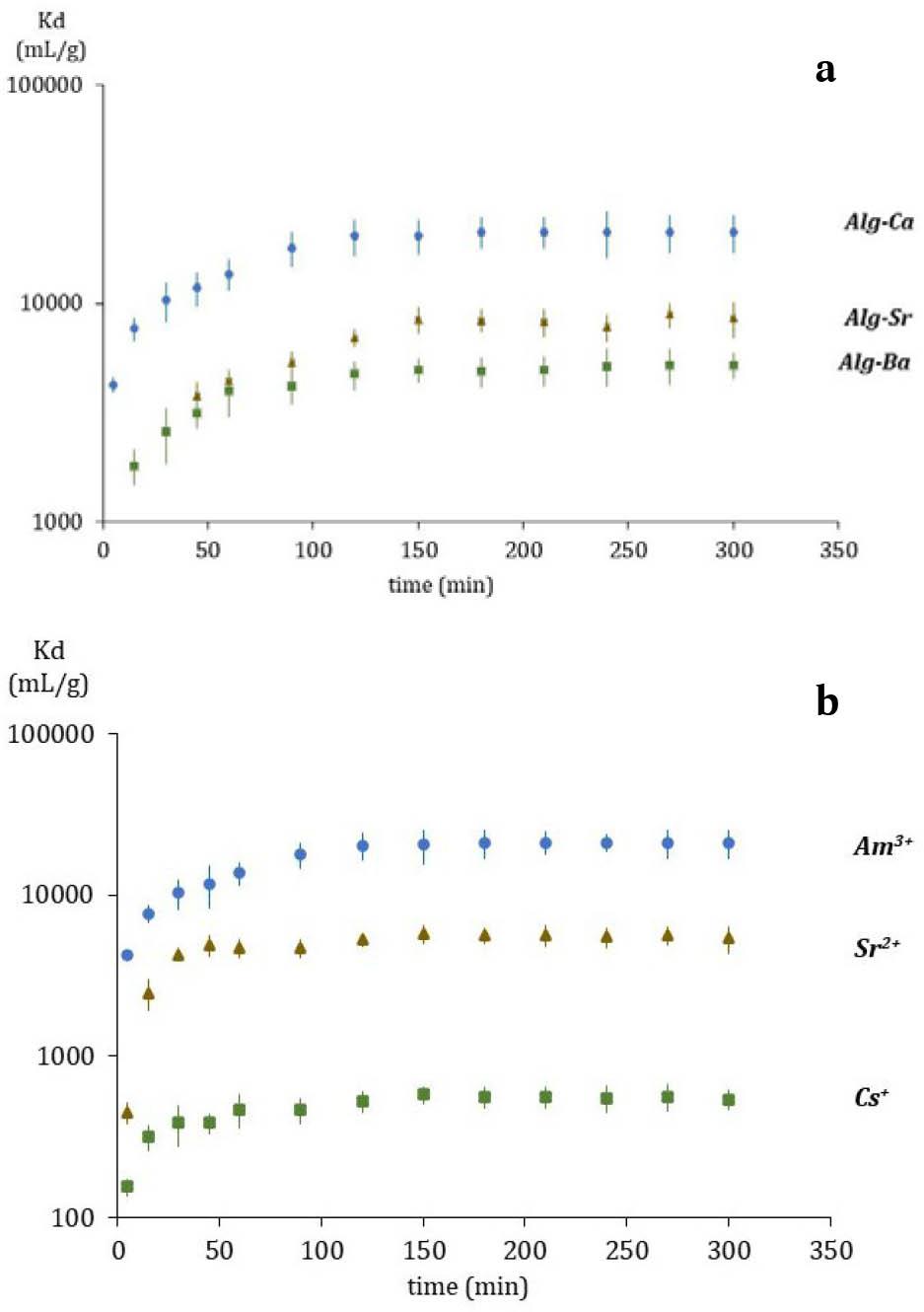
Fig. 2
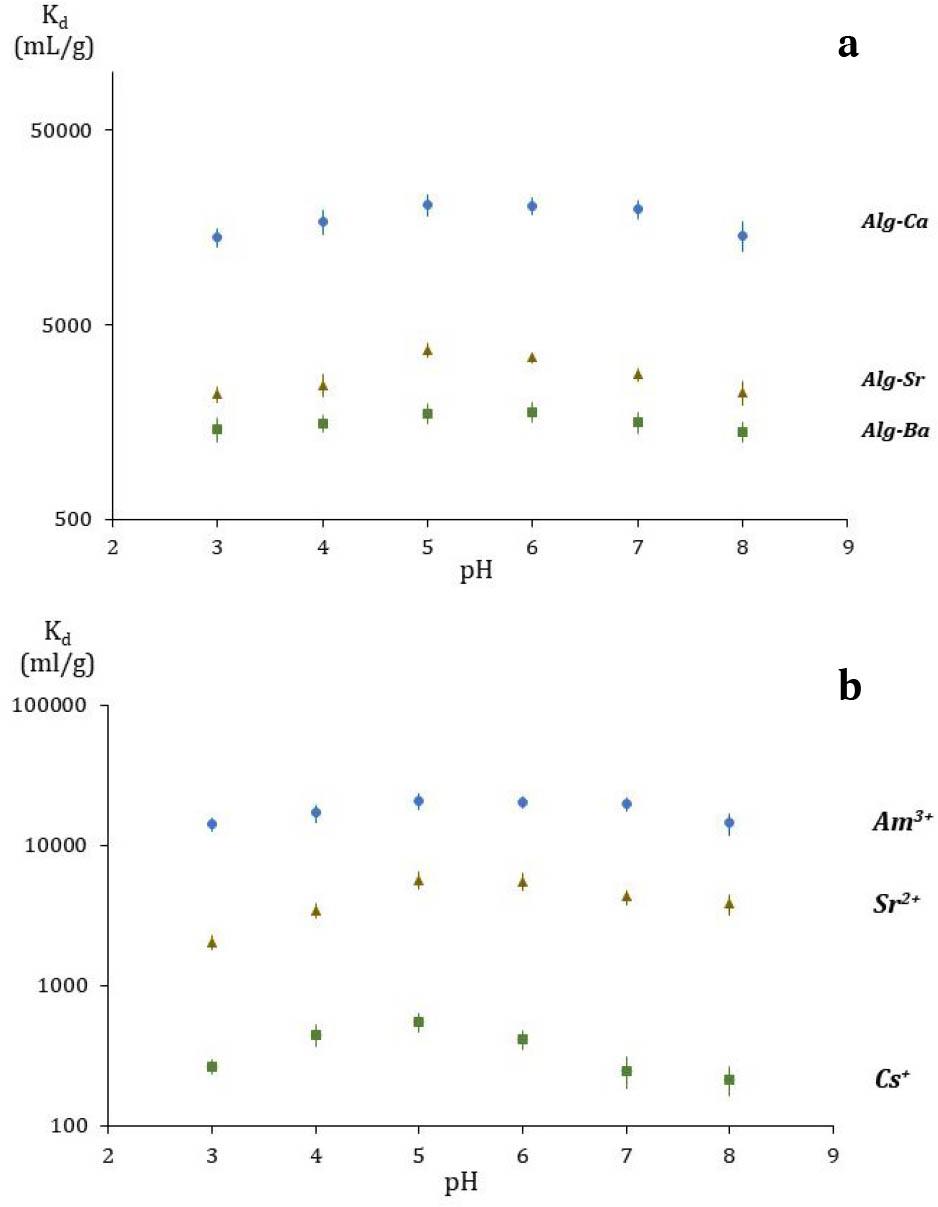
Fig. 3
![Am3+ complexes in aqueous solutions of different pH. Simulation computed using the Medusa program [11].](https://sciendo-parsed.s3.eu-central-1.amazonaws.com/647260fc215d2f6c89dc6350/j_nuka-2021-0023_fig_003.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Credential=AKIA6AP2G7AKOUXAVR44%2F20251128%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20251128T130630Z&X-Amz-Expires=3600&X-Amz-Signature=cab9ba5299bf7cabdf92846cadd55221382d947e8dac41b5c8cb52430237eebf&X-Amz-SignedHeaders=host&x-amz-checksum-mode=ENABLED&x-id=GetObject)
Fig. 4

Parameters of Freundlich, Langmuir and Dubinin–Radushkevich isotherms of Cs+, Sr2+ and Am3+ sorption on calcium, strontium and barium alginate sorbents (pH 5, time: 4 h, temperature: 21 ± 1°C)
| Freundlich isotherm | Langmuir isotherm | Dubinin–Radushkevich isotherm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KF (dm3/mg) | 1/n | R2 | amax | KL (dm3/mg) | R2 | Xm (mg/g) | B (mol2/J2) | E (kJ/mol) | R2 | |
| Calcium alginate | ||||||||||
| Am(III) | 2.87 × 10−5 | 0.97 | 0.99 | 4.46 × 10−3 | 8.98 × 102 | 0.80 | 0.02 | 1.41 × 10−4 | 7.07 | 1.00 |
| Sr(II) | 4.68 × 10−7 | 1.00 | 1.00 | 2.99 × 10−3 | 5.00 × 102 | 0.77 | 0.02 | 1.41 × 10−4 | 7.07 | 1.00 |
| Cs(I) | 1.67 × 10−7 | 1.00 | 1.00 | 1.23 × 10−3 | 6.23 × 101 | 0.90 | 0.01 | 1.41 × 10−4 | 7.07 | 1.00 |
| Strontium alginate | ||||||||||
| Am(III) | 3.54 × 10−8 | 1.46 | 0.90 | 6.57 × 10−2 | 5.70 × 102 | 0.84 | 0.01 | 1.41 × 10−4 | 7.07 | 1.00 |
| Sr(II) | 2.50 × 10−6 | 1.05 | 1.00 | 2.99 × 10−3 | 2.27 × 102 | 0.96 | 0.01 | 1.41 × 10−4 | 7.07 | 1.00 |
| Cs(I) | 1.55 × 10−5 | 1.00 | 1.00 | 1.31 × 10−2 | 6.57 × 101 | 0.84 | 0.02 | 1.41 × 10−4 | 7.07 | 1.00 |
| Barium alginate | ||||||||||
| Am(III) | 3.17 × 10−6 | 1.34 | 0.91 | 6.75 × 10−2 | 7.70 × 102 | 0.83 | 0.01 | 1.41 × 10−4 | 7.07 | 1.00 |
| Sr(II) | 7.32 × 10−6 | 0.99 | 1.00 | 2.86 × 10−3 | 1.47 × 102 | 0.80 | 0.01 | 1.41 × 10−4 | 7.07 | 1.00 |
| Cs(I) | 1.67 × 10−5 | 1.00 | 1.00 | 1.30 × 10−2 | 6.31 × 101 | 0.83 | 0.02 | 1.41 × 10−4 | 7.07 | 1.00 |
The desorption percentage of Cs+, Sr2+ and Am3+ from the alginates studied_ Standard deviation in the population of each determination: about ±0_1%
| H2O | HCl | NaNO3 | NaHCO3 | Na2SO4 | |
|---|---|---|---|---|---|
| Calcium alginate | |||||
| Am3+ | 1.6% | 4.4% | 0.9% | 7.8% | 8.7% |
| Sr2+ | 5.5% | 12.5% | 8.8% | 9.9% | 5.5% |
| Cs+ | 2.2% | 2.2% | 2.2% | 2.2% | 2.2% |
| Strontium alginate | |||||
| Am3+ | 0.8% | 1.0% | 3.3% | 7.3% | 10.8% |
| Sr2+ | 6.1% | 15.8% | 15.8% | 30.4% | 25.5% |
| Cs+ | 2.4% | 2.4% | 2.4% | 2.4% | 2.4% |
| Barium alginate | |||||
| Am3+ | 0.5% | 1.6% | 2.0% | 7.6% | 8.9% |
| Sr2+ | 8.3% | 25.0% | 17.6% | 15.9% | 7.3% |
| Cs+ | 2.4% | 2.4% | 2.4% | 2.4% | 2.4% |