Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
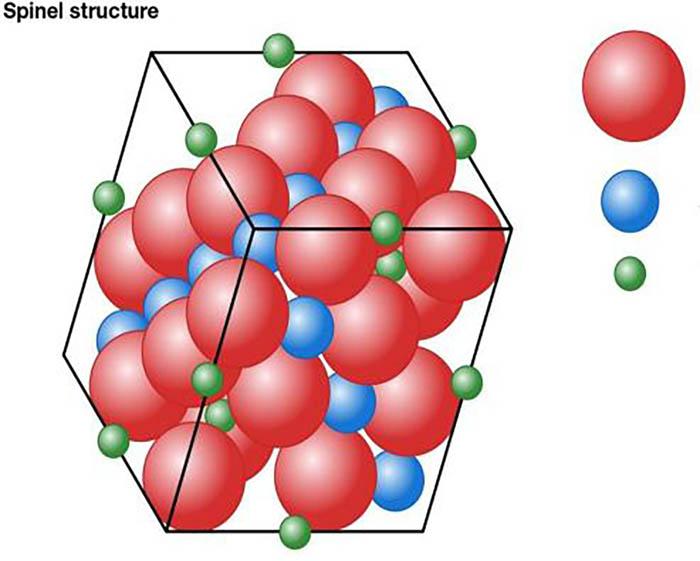
Figure 6
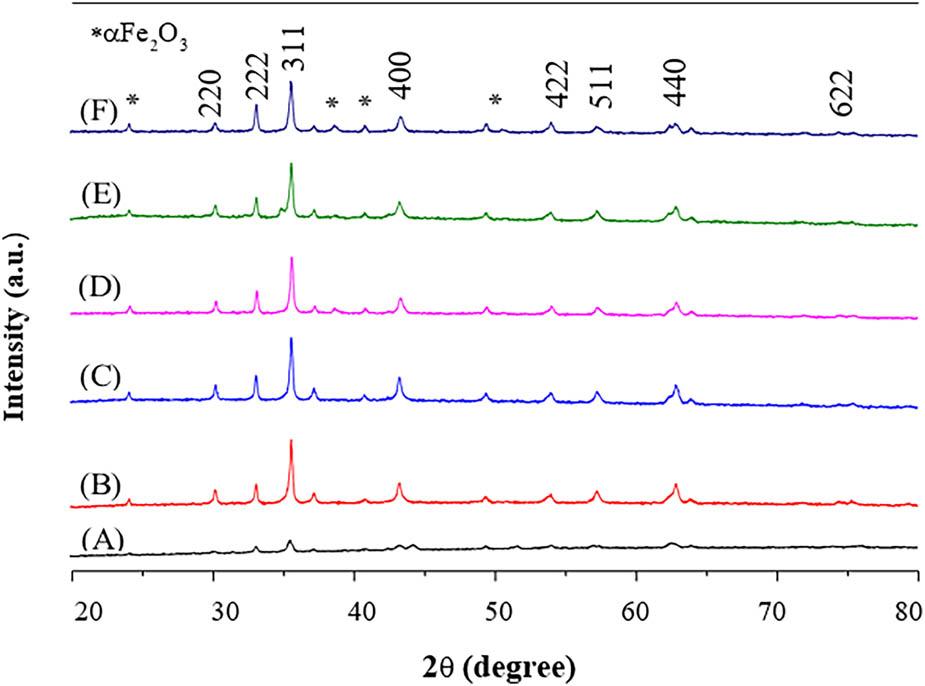
Figure 7
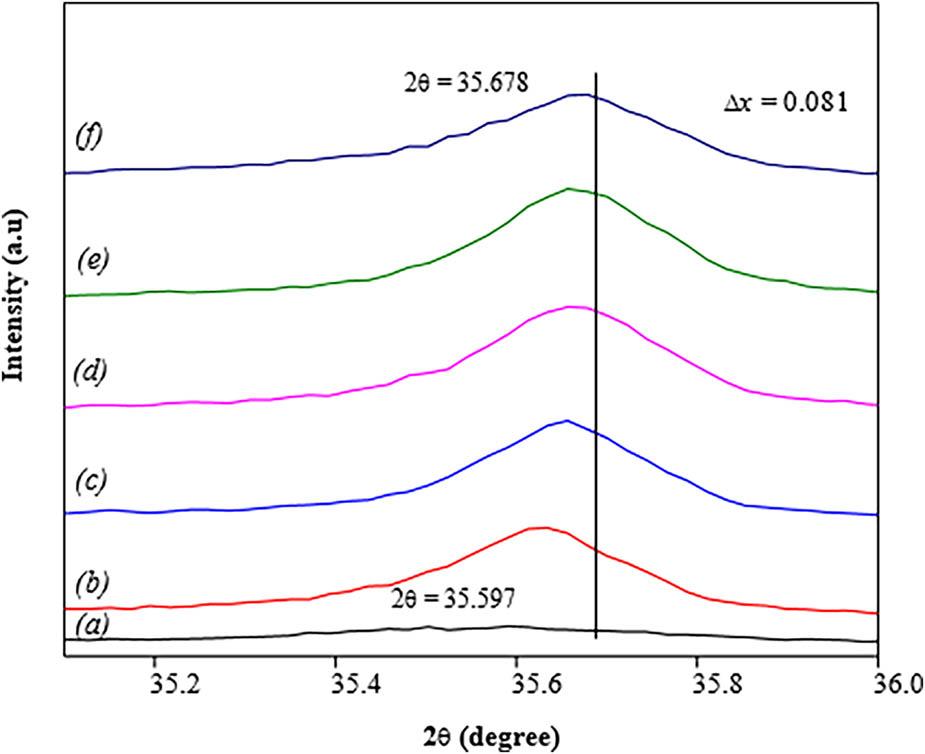
Figure 8
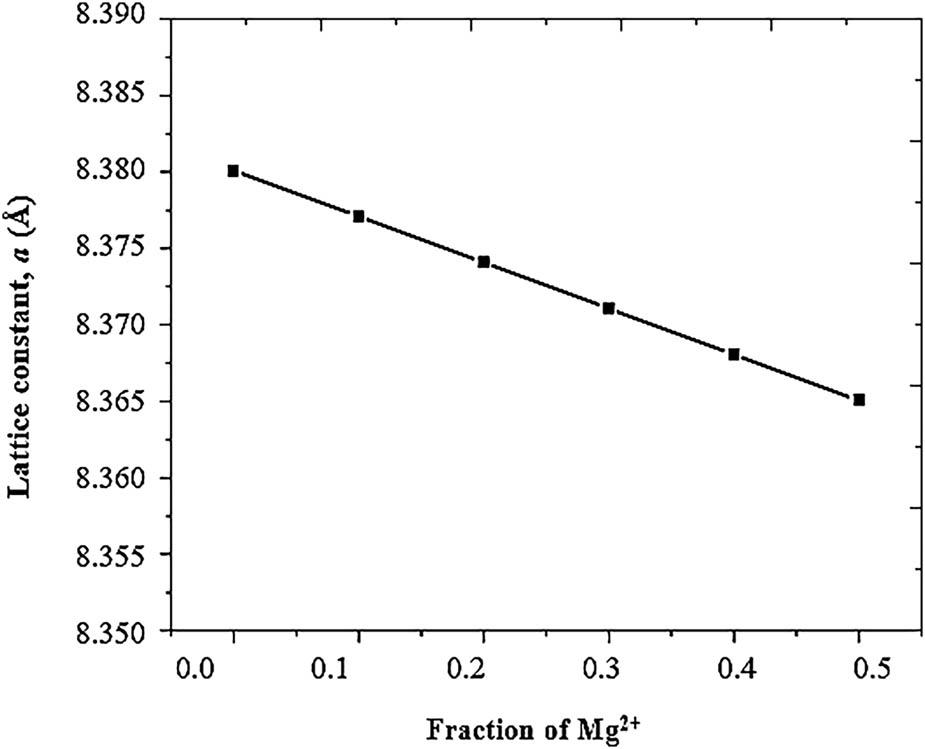
Figure 9
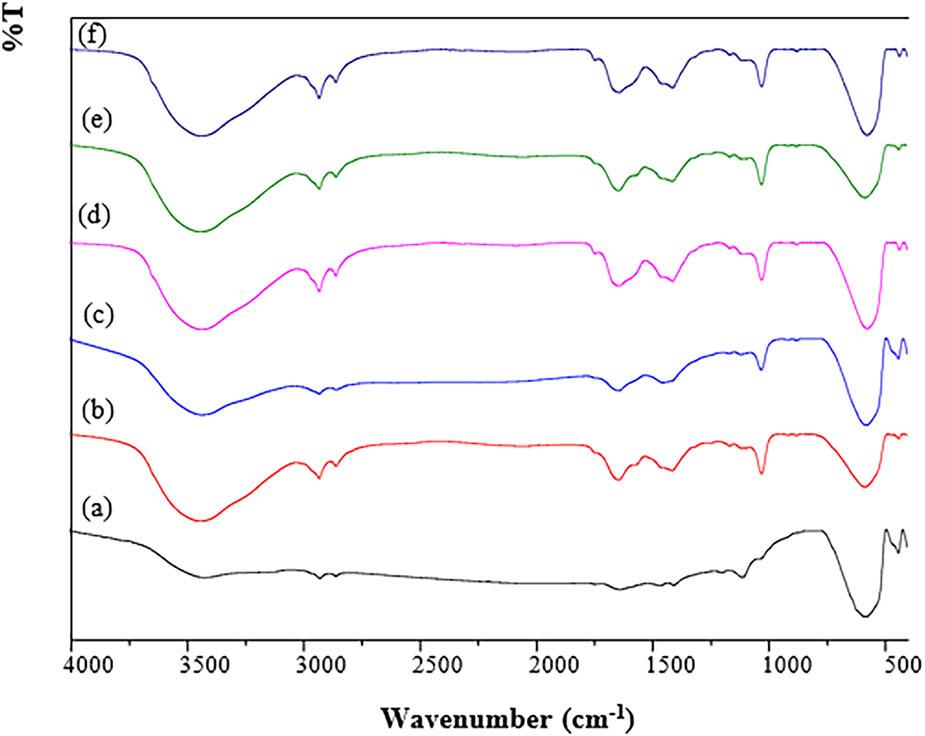
Figure 10
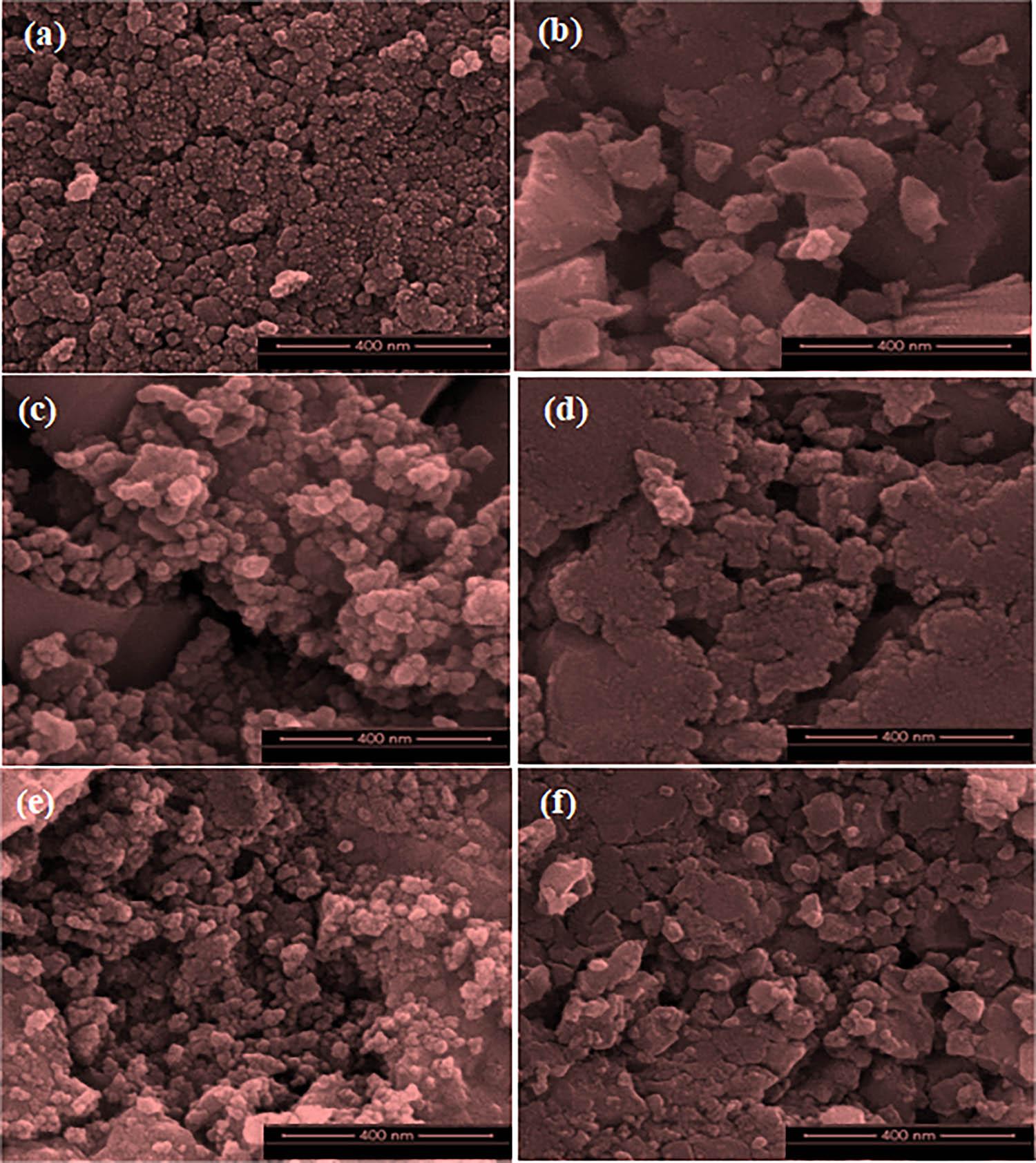
Figure 11
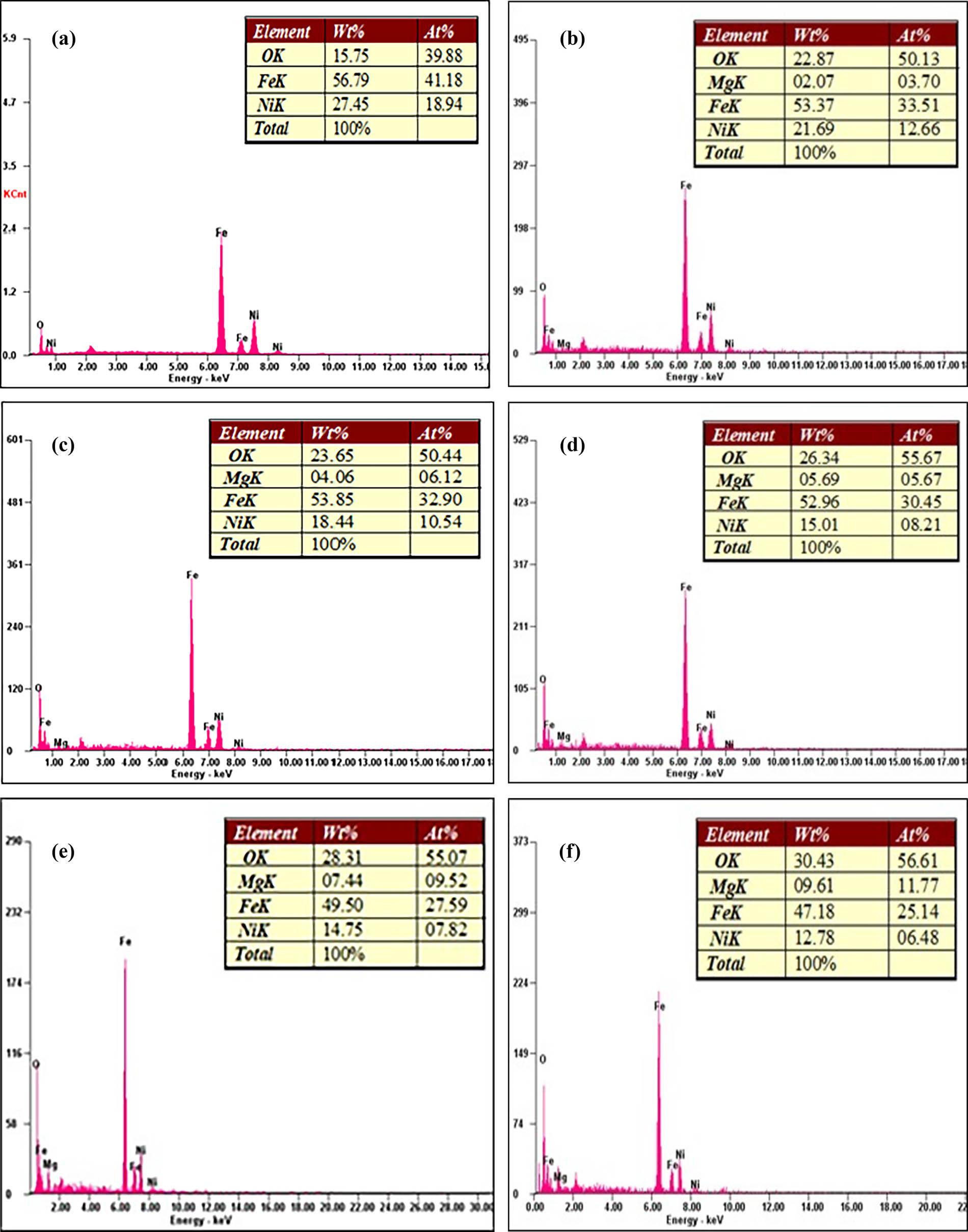
Figure 12
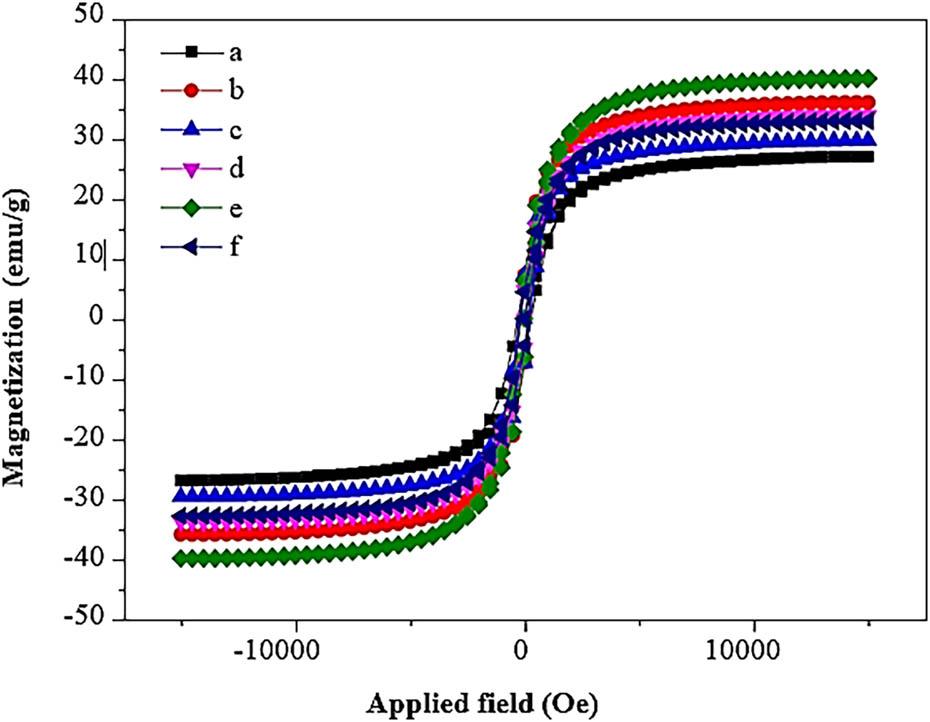
Figure 13
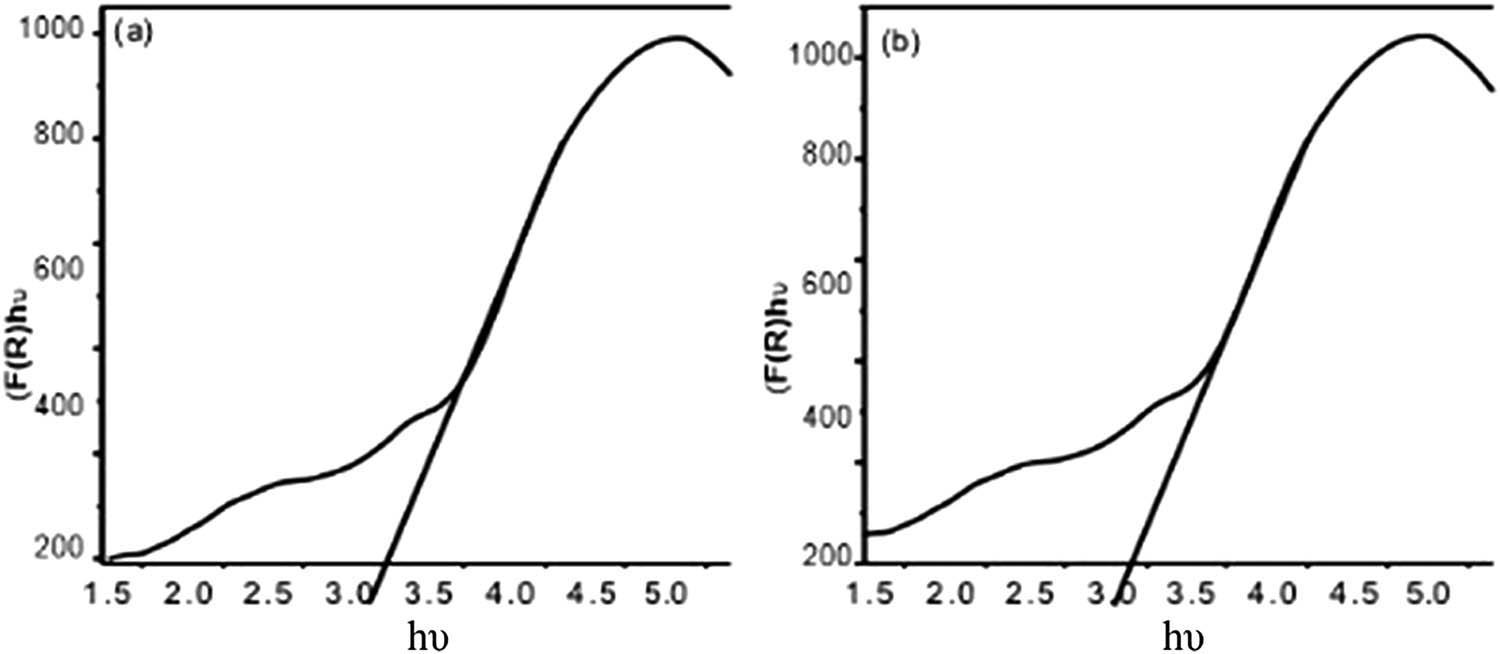
Figure 14
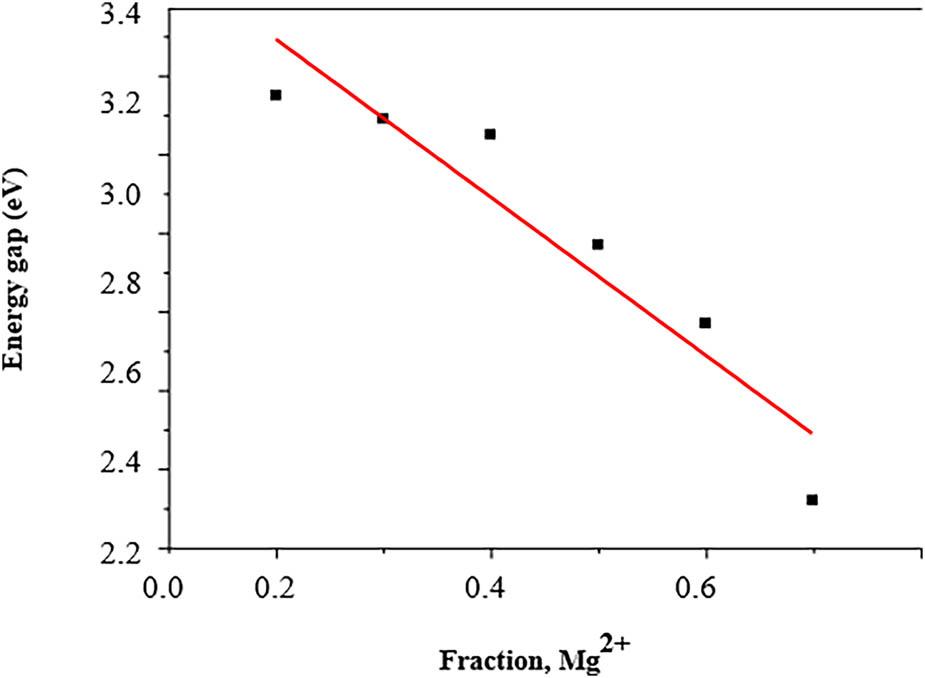
Hc, Mr, and Ms values of Ni1–x Mg x Fe2O4 (0 ≤ x ≤ 0_5) nanoparticles_
| Sample code | Hc (Oe) | Mr (emu/g) | Ms (emu/g) |
|---|---|---|---|
| A | 337.01 | 5.21 | 17.37 |
| B | 502.14 | 5.45 | 18.24 |
| C | 171.11 | 5.54 | 20.94 |
| D | 217.08 | 6.17 | 22.35 |
| E | 195.29 | 7.35 | 25.79 |
| F | 158.77 | 6.52 | 24.72 |
Sample code, crystallite size, lattice parameter, and band gap values of Ni1−x Mg x Fe2O4 (0 ≤ x ≤ 0_5) samples_
| Sample | Sample code | L (nm) | D (nm) | a (Å) | Eg (eV) |
|---|---|---|---|---|---|
| NiFe2O4 | A | 20 | 20 | 8.380 | 3.35 |
| Ni0.9Mg0.1Fe2O4 | B | 22 | 21 | 8.377 | 3.29 |
| Ni0.8Mg0.2Fe2O4 | C | 24 | 23 | 8.374 | 3.25 |
| Ni0.7Mg0.3Fe2O4 | D | 27 | 25 | 8.371 | 2.97 |
| Ni0.6Mg0.4Fe2O4 | E | 29 | 27 | 8.368 | 2.73 |
| Ni0.5Mg0.5Fe2O4 | F | 31 | 30 | 8.365 | 2.32 |