Fig. 1
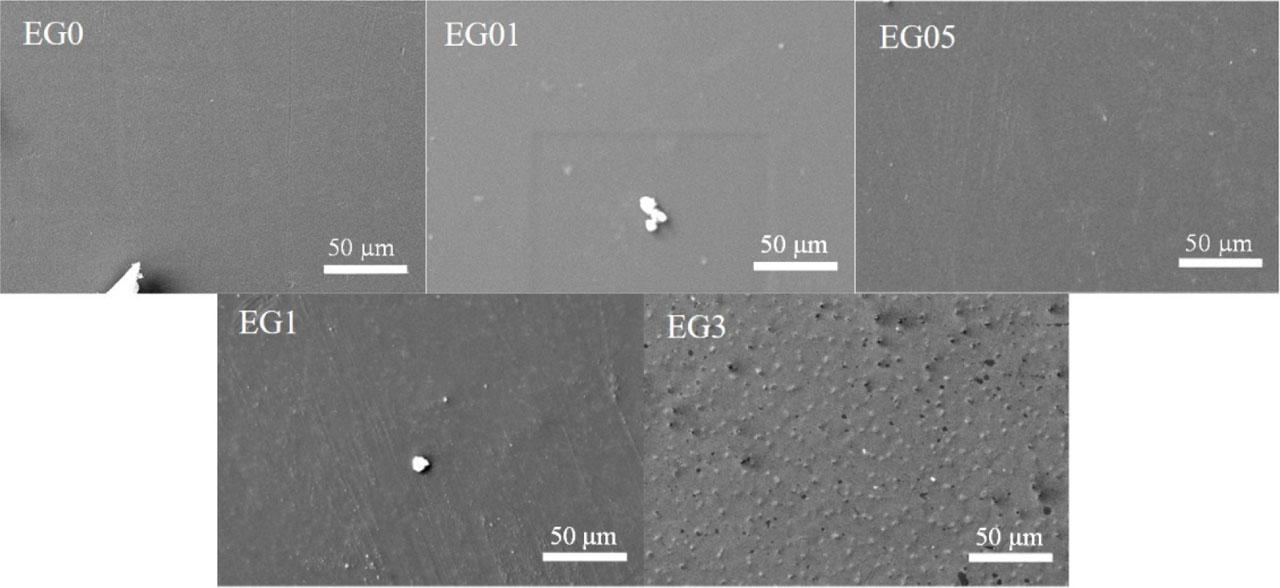
Fig. 2
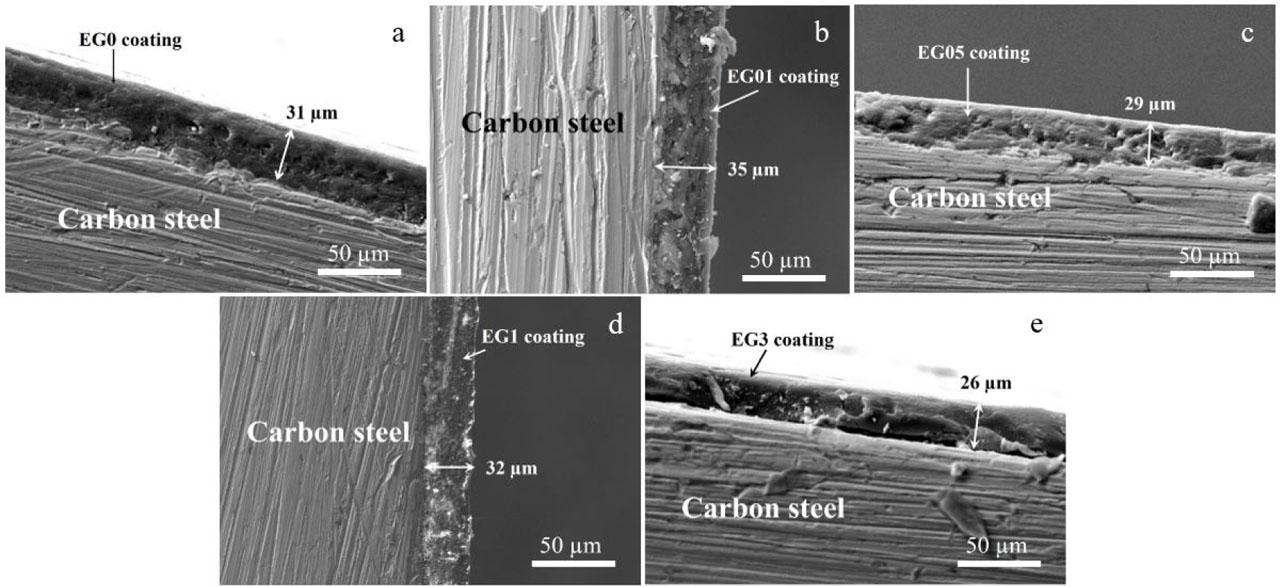
Fig. 3
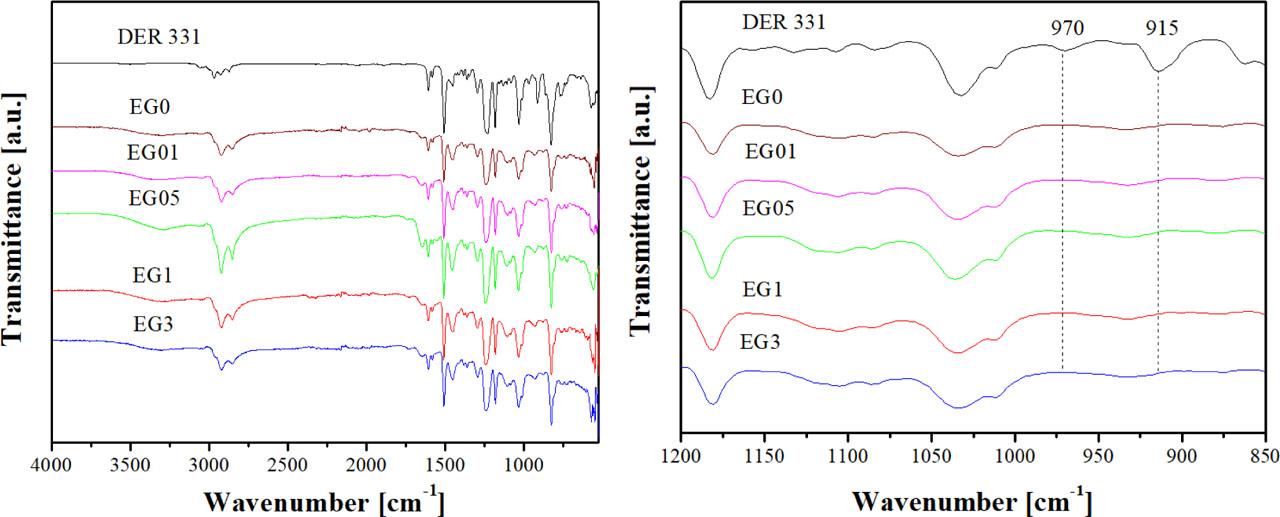
Fig. 4

Fig. 5
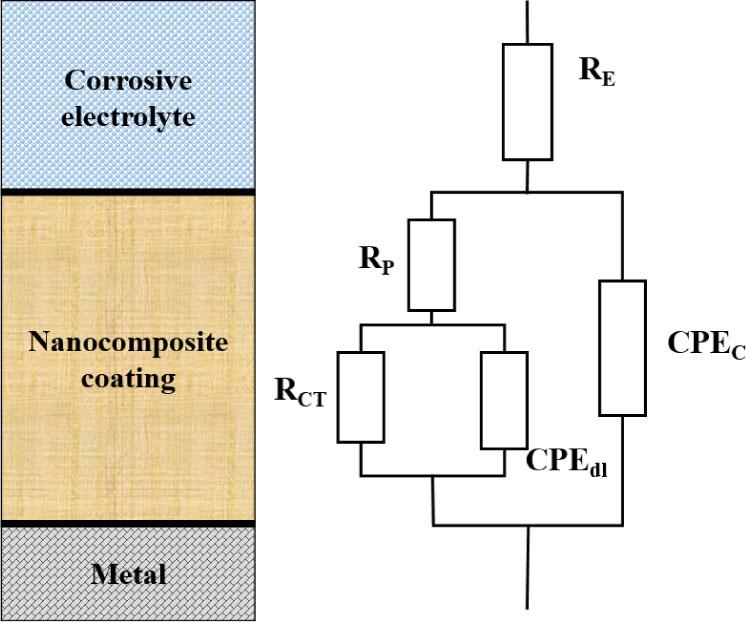
Fig. 6
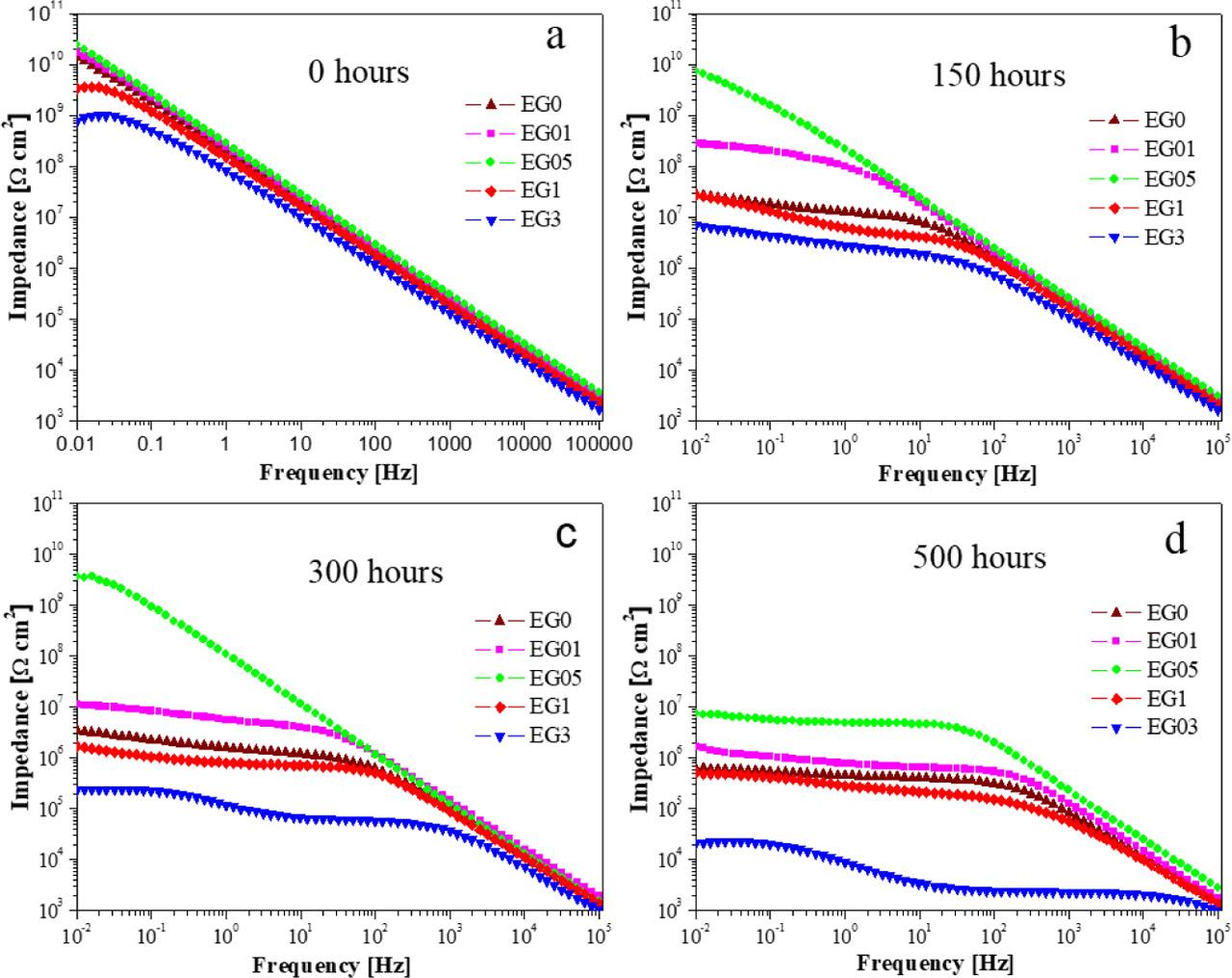
Fig. 7

Fig. 8

The electrochemical parameters extracted from EIS data for nanocomposite epoxy coatings containing 0 wt_%, 0_1 wt_%, 0_5 wt_%, 1 wt_%, and 3 wt_% GO after immersion in 5 wt_% NaCl solution for different durations
| Sample | time (hours) | CPEC | RP (Ω·cm2) | CPEdl | RCT (Ω·cm2) | x2 | ||
|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·sn) | n | Y0 (Ω−1·cm−2·sn) | n | |||||
| EG0 | 0 | 8.70E−10 | 9.77E−01 | 4.89E+10 | 7.11E−10 | 9.52E−01 | 1.45E+10 | 4.64E−04 |
| 150 | 2.32E−09 | 9.56E−01 | 9.80E+06 | 1.37E−07 | 4.00E−01 | 6.07E+08 | 2.42E−04 | |
| 300 | 1.96E−09 | 9.15E−01 | 9.50E+06 | 7.44E−07 | 2.89E−01 | 2.36E+07 | 3.96E−04 | |
| 500 | 5.79E−09 | 8.71E−01 | 321000 | 4.67E−06 | 5.61E−01 | 8.71E+05 | 2.59E−03 | |
| EG01 | 0 | 6.96E−10 | 9.66E−01 | 5.45E+10 | 2.92E−10 | 6.00E−01 | 1.49E+12 | 2.39E−03 |
| 150 | 1.80E−09 | 9.34E−01 | 8.24E+07 | 5.53E−09 | 8.40E−01 | 4.64E+08 | 4.40E−03 | |
| 300 | 2.38E−09 | 9.51E−01 | 3.90E+07 | 1.77E−07 | 3.97E−01 | 1.12E+07 | 2.82E−04 | |
| 500 | 4.46E−09 | 9.38E−01 | 431000 | 1.82E−06 | 2.63E−01 | 5.58E+08 | 9.06E−04 | |
| EG05 | 0 | 7.97E−10 | 9.82E−01 | 9.60E+10 | 1.72E−09 | 8.23E−01 | 5.30E+09 | 6.68E−04 |
| 150 | 9.28E−10 | 9.77E−01 | 8.73E+09 | 3.53E−10 | 4.35E−01 | 1.38E+12 | 3.56E−04 | |
| 300 | 7.13E−10 | 9.69E−01 | 7.26E+09 | 8.56E−10 | 6.19E−01 | 1.27E+10 | 4.45E−04 | |
| 500 | 9.13E−10 | 9.59E−01 | 4.70E+09 | 2.66E−11 | 1.80E−01 | 6.21E+07 | 1.08E−02 | |
| EG1 | 0 | 1.06E−09 | 9.70E−01 | 5.29E+09 | 1.34E−13 | 9.59E−01 | 6.32E+09 | 5.01E−03 |
| 150 | 2.97E−09 | 8.99E−01 | 3.94E+06 | 1.62E−07 | 6.20E−01 | 2.96E+10 | 8.81E−02 | |
| 300 | 3.14E−09 | 8.96E−01 | 1.54E+07 | 2.36E−06 | 4.46E−01 | 5.84E+06 | 7.93E−04 | |
| 500 | 7.05E−09 | 9.57E−01 | 138000 | 3.62E−06 | 4.12E−01 | 4.11E+06 | 3.24E−04 | |
| EG3 | 0 | 2.41E−09 | 8.13E−01 | 3.16E+09 | 2.42E−09 | 9.23E−01 | 1.30E+09 | 2.31E−02 |
| 150 | 4.18E−09 | 9.19E−01 | 1.32E+06 | 3.35E−07 | 3.00E−01 | 3.38E+07 | 4.36E−04 | |
| 300 | 1.50E−08 | 8.80E−01 | 22330 | 5.37E−06 | 7.99E−01 | 1.89E+05 | 1.56E−03 | |
| 500 | 2.48E−08 | 8.54E−01 | 2270 | 2.81E−05 | 7.46E−01 | 2.22E+04 | 1.91E−03 | |