Fig. 1.
Fig. 2.
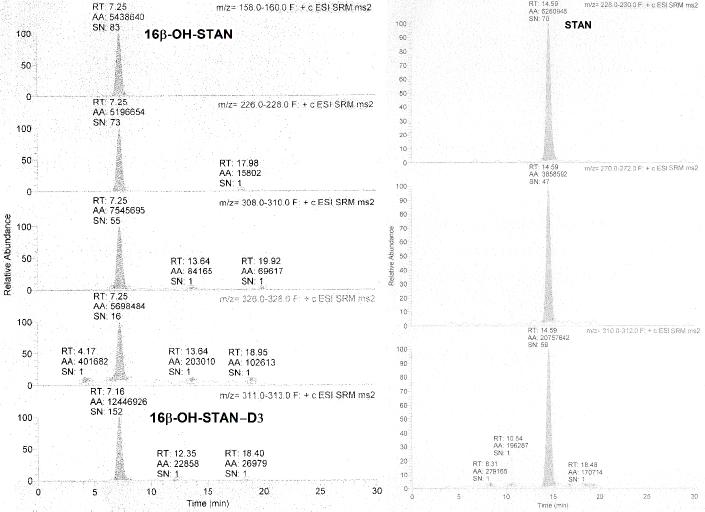
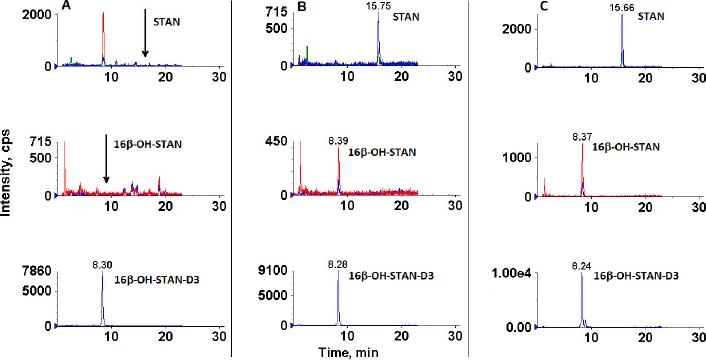
Fig. 3.
| LC-MS2 (IT) | ||||
|---|---|---|---|---|
| Compound | SRM transition (m/z) | Collision energy CE (%) | Ion ratio average ± SD | Samples fulfilling the confirmation criteria (%)* |
| 1.00-10.00 μg L−1 | ||||
| STAN | 329>311a | 35 | - | - |
| 329>271 | 0.228 ± 0.032 | 75.3 | ||
| 329>229 | 0.363 ± 0.067 | 74.1 | ||
| 16β-OH-STAN | 345>327 | 37 | 0.653 ± 0.212 | 51.9 |
| 345>309 | - | - | ||
| 345>227 | 0.734 ± 0.163 | 55.6 | ||
| 345>159 | 0.735 ± 0.135 | 58.0 | ||
| 16β-OH-STAN-D3 | 348>312 | 36 | - | - |
Validation parameters of the liquid chromatography-tandem triple quadrupole (LC-MS/MS (QqQ) and -tandem ion-trap (LC-MS2 (IT) mass spectrometry methods for the determination of stanozolol (STAN) and its metabolite 16β-OH-stanozolol (16β-OH-STAN) in bovine urine
| Number of samples | Spiking level (μg L−1) | Compound | ||||
|---|---|---|---|---|---|---|
| STAN* | 16β-OH-STAN* | STAN** | 16β-OH-STAN** | |||
| Apparent recovery (%) | n=18*/21** | 1.00*/0.50** | 125.8 | 85.4 | 64.5 | 98.8 |
| 2.00/1.00 | 104.7 | 101.7 | 65.9 | 94.0 | ||
| 3.00/1.50 | 88.4 | 90.7 | 66.8 | 88.7 | ||
| n = 18*/6** | 4.00/2.00 | 87.4 | 94.3 | 98.8 | 103.2 | |
| 10.00/5.00 | 81.1 | 88.0 | 96.4 | 97.1 | ||
| n= 6** | CCα** | NE | NE | 91.9 | 114.6 | |
| Repeatability (RSD, %) | n=18*/21** | 1.00*/0.50** | 22.4 | 24.0 | 11.2 | 4.4 |
| 2.00/1.00 | 22.7 | 19.2 | 6.8 | 6.1 | ||
| 3.00/1.50 | 12.9 | 17.5 | 7.3 | 4.4 | ||
| 4.00/2.00 | 20.9 | 11.9 | 6.7 | 13.4 | ||
| n = 18*/6** | 10.00/5.00 | 27.7 | 13.6 | 7.9 | 12.1 | |
| n = 6** | CCα** | NE | NE | 15.3 | 10.7 | |
| Within-lab reproducibility (RSD, %) | n=18*/21** | 2.00*/0.50** | 27.2 | 22.9 | 23.8 | 10.5 |
| 3.00/1.00 | 22.0 | 17.6 | 15.2 | 17.7 | ||
| 4.00/1.50 | 30.2 | 12.3 | 10.9 | 10.9 | ||
| Decision limit (CCα, μg L−1) | 0.44 | 0.25 | 0.14 | 0.08 | ||
| Detection capability (CCβ, μg L−1) | 0.75 | 0.42 | 0.24 | 0.13 | ||
| Measurement uncertainty at validation level of *2 μg L−1/**1 μg L−1 (U, k = 2, μg L−1/ %) | 0.37/18.5 | 0.31/15.5 | 0.20/20.0 | 0.34/34.0 | ||
| Matrix effect (ME, %) | NE | NE | 7.0 | 10.0 | ||
| Standard calibration curve | ||||||
| Slope ± sb | 0.2119 ± 0.0843 | 0.1058 ± 0.2351 | 0.2112 ± 0.2456 | 0.1443 ± 0.0845 | ||
| y-Intercept ± sa | 0.0812 ± 0.0838 | −0.0027 ± 0.0737 | 0.0570 ± 0.0424 | 0.0125 ± 0.0096 | ||
| Correlation coefficient | 0.9945 | 0.9985 | 0.9866 | 0.9970 | ||
| Standard error | 0.0662 | 0.0127 | 0.0884 | 0.0285 | ||
| Matrix matched calibration curve | ||||||
| Slope ± sb | 0.1781 ± 0.1220 | 0.1228 ± 0.0223 | 0.2516 ± 0.1005 | 0.1564 ± 0.0554 | ||
| y-Intercept ± sa | 0.1656 ± 0.1199 | −0.0102 ± 0.0088 | −0.0322 ± 0.0291 | −0.0060 ± 0.0103 | ||
| Correlation coefficient | 0.9983 | 0.9995 | 0.0291 | 0.9988 | ||
| Standard error | 0.0426 | 0.0156 | 0.0582 | 0.0148 | ||
| Additional series of validation** | ||||||
| Compound | Number of samples | Spiking level (μg L−1) | STAN | 16β-OH-STAN | ||
| Apparent recovery (%)// | n = 7 | 0.25 | 89.4//3.1 | 102.7//6.7 | ||
| 0.50 | 70.2//4.4 | 108.3//1.8 | ||||
| 0.75 | 79.8//10.2 | 101.7//4.5 | ||||
| Repeatability (RSD, %) | n = 4 | 1.00 | 80.5//5.5 | 100.2//4.7 | ||
| 2.00 | 86.8//15.9 | 96.5//3.1 | ||||
| 5.00 | 81.4//9.0 | 94.0//2.9 | ||||
| n = 10 | CCα | 105.0//4.9 | 101.3//20.0 | |||
| Decision limit (CCα, μg L−1) | 0.27 | 0.28 | ||||
| Standard curve: Equation/Correlation coefficient | y = 0.6440x-0.0664/0.9998 | y = 0.1570x-0.0168/0.9971 | ||||
| Matrix matched calibration curve: Equation/Correlation coefficient | y = 0.5230x-0.0670/0.9997 | y = 0.1453x-0.0033/1.0000 | ||||
Liquid chromatography-tandem mass spectrometry ion acquisition parameters used for the identification of stanozolol (STAN) and 16β-OH-stanozolol (16β-OH-STAN) using a 16β-OH-stanozolol-D3 internal standard (16β-OH-STAN-D3)
| LC-MS/MS (QqQ) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | MRM transition (m/z) | Collision energy CE (V) | Declustering potential DP (V) | Entrance potential EP (V) | Collision cell exit potential CXP (V) | Ion ratio average ± SD | Samples fulfilling the confirmation criteria (%)* | |
| 0.50-5.00 μg L−1 | CCα level | |||||||
| STAN | 329>121 | 52 | 212 | 10 | 10 | 0.346 ± 0.023 | 98.7 | 100.0 |
| 329>95 | 54 | 0.395 ± 0.019 | 100.0 | 100.0 | ||||
| 329>81a | 68 | - | - | - | ||||
| 16β-OH-STAN | 345>95 | 61 | 214 | 10 | 10 | 0.399 ± 0.027 | 100.0 | 100.0 |
| 345>81 | 70 | - | - | - | ||||
| 16β-OH-STAN-D3 | 348>81 | 72 | 198 | 10 | 5 | - | - | - |
| Additional series of validation | Ion ratio average ± SD | Samples fulfilling the confirmation criteria (%)** | ||||||
| 0.25-5.00 μg L−1 | CCα level | |||||||
| STAN | 329>121 | as above | 0.218 ± 0.028 | 100.0 | 100.0 | |||
| 329>95 | 0.273 ± 0.009 | 100.0 | 100.0 | |||||
| 329>81a | - | - | - | |||||
| 16β-OH-STAN | 345>95 | as above | 0.264 ± 0.039 | 100.0 | 100.0 | |||
| 345>8l | - | - | - | |||||