Mackerels of the genus Trachurus Rafinesque, 1810, are marine fish belonging to the family Carangidae Rafinesque, 1815. This family is highly diverse and abundant in tropical and subtropical seas, encompassing around 33 genera and 146 species. Among them, the genus Trachurus comprises 14 valid species found in oceans worldwide, except Arctic and Antarctic waters (Costa et al., 2017; Nasri et al., 2024). The representatives of this genus can be easily recognized from other Carangidae by the presence of two lateral lines running along their bodies, which serve as sensory organs (Nasri et al., 2024). In the Mediterranean Sea, the genus Trachurus is represented by three species: the horse mackerel (Trachurus trachurus Linnaeus, 1758), the Mediterranean horse mackerel (Trachurus mediterraneus Steindachner, 1868), and the blue jack mackerel (Trachurus picturatus Bowdich, 1825). All three species are commercially exploited (Gonçalves et al., 2013), with T. trachurus being the most abundant, economically significant, and widely consumed worldwide (Abaunza et al., 2003; Costa et al., 2021). The horse mackerel, Trachurus trachurus, is a semi-pelagic species with a cosmopolitan distribution and a migratory lifestyle, inhabiting the Mediterranean, northeastern Atlantic, and the Black Sea (Jardas et al., 2004; Bektaş & Belduz, 2009; Costa et al., 2017). As a key species in pelagic and demersal fisheries (Bayhan & Sever, 2009), it has been the subject of numerous studies, particularly regarding its Digenean parasite diversity in the Black Sea (Paradižnik & Radujković, 2007; Öztürk & Özer, 2016), the Atlantic (MacKenzie et al., 2004; MacKenzie et al., 2008), and the Mediterranean, where twelve digenean species were identified in its digestive tract. These include: Tergestia laticollis (Rudolphi, 1819) Stossich, 1899, Lecithochirium grandiporum (Rudolphi, 1819) Lühe, 1901, Lecithocladium excisum (Rudolphi, 1819) Lühe, 1901, Hemiurus communis Odhner, 1905, Ectenurus lepidus Looss, 1907, Monascus filiformis (Rudolphi, 1819) Looss, 1907, Ancylocoelium typicum Nicoll, 1912, Opechona bacillaris (Molin, 1859) Dollfus, 1927, Bathycreadium elongatum (Maillard, 1970) Bray, 1973, Prodistomum polonii (Molin, 1859) Bray & Gibson, 1990, Pseudopecoeloides chloroscombri (Fischthal & Thomas, 1970) Bartoli, Bray & Gibson, 2003 and Opechona sp. (Mazza, 1963; Amine, 1999; Bartoli et al., 2003; Bartoli & Bray, 2004; MacKenzie et al., 2004; Bartoli et al., 2005; MacKenzie et al., 2008; Derbel et al., 2012; Abid-Kachour, 2014; Feki et al., 2016; Ichalal et al., 2017). However, despite its ecological and economic significance, the Digenea diversity of T. trachurus in Algerian waters remains poorly explored (Amine, 1999; Abid-Kachour, 2014; Ichalal et al., 2017). In light of this gap, and building on our previous research efforts (Gharbi et al., 2023; Boukadoum & Tazerouti, 2024; Boukadoum et al., 2024; Zedam et al., 2024), we aimed to expand the knowledge on Digenean parasites infesting T. trachurus along the Algerian coastline by providing the first comprehensive assessment of their host–parasite dynamics in this region. We sought to offer new insights into species diversity while also examining how infestation levels vary between seasons and regions. To achieve this, we calculated key parasitological parameters, including prevalence, mean intensity, and mean abundance. Additionally, we assessed whether host morphometric traits (total length and weight) influence parasite load. Statistical analyses, specifically the Chisquare test, the Kruskal–Wallis test, and Spearman correlation, were applied to explore these patterns.
Between 2023 and 2024, a total of 820 T. trachurus specimens (8.5 – 49 cm in total length, 9 – 328.5 g in weight) were collected from various sites along the Algerian coast. Sampling locations covered the western region, with Ghazaouet (35°06'0" N, 1°51'0" W); the central region, comprising Cherchell (36°36'31" N, 2°11'50" E), Tipaza (36°35'31" N, 2°26'58" E), Bouharoun (36°37'24" N, 2°39'17" E), Algiers (36°45'8" N, 3°02'31" E), Tamentfoust (36°48'19.962" N, 3°13'47.193" E), Reghaïa (36°44'00" N, 3°21'00" E), Boudouaou El Bahri (36° 46’ 38.232” N, 3° 23’ 1.975” E), Zemmouri El Bahri (36° 48’ 11.596” N, 3° 33’ 39.165” E), Cap Djinet (36°52'37" N, 3°43'23" E), Dellys (36°54'48" N, 3°54'51" E); and the eastern region, represented by Béjaïa (36°45'00" N, 5°04'00" E), Jijel (36°49'14" N, 5°46'0.2" E) and Annaba (36°54'27" N, 7°45'26" W) (Fig. 1). The collected fish specimens were immediately transported to the Laboratory of Biodiversity and Environment: Interactions-Genomes, USTHB for identification using the taxonomic keys of Fischer et al. (1987). The digestive tracts were then carefully dissected and examined under a stereomicroscope to detect the presence of digenean parasites.
Map of the Algerian sample collection sites. Main fishing ports along the Algerian coast (modified map).
All collected Digenea were slightly flattened between a slide and cover glass before being fixed for 3 – 5 minutes with Bouin-Hollande fixative and subsequently stored in 70 % ethanol. They were then stained with boracic carmine, dehydrated through a graded ethanol series with increasing concentrations (70 %, 96 %, and 100 %), cleared in clove oil, and mounted in Canada balsam.
For each Digenea species, the prevalence (P %), mean abundance (MA), and mean intensity (MI) were calculated following Margolis et al. (1982) and Bush et al. (1997). To analyze statistical differences in parasite prevalence across seasons and regions, the Chisquare (X2) test was applied. Additionally, the Kruskal-Wallis test was used to assess variations in parasite abundance based on these factors. To further investigate the relationship between host morphometric traits and parasitism, Sturges’ rule was employed to categorize hosts by weight and length. Subsequently, Spearman’s correlation test was performed to evaluate the influence of host weight and total length on the mean abundance and intensity of each parasite species. All statistical analyses were conducted using IBM SPSS Statistics (Version 26), with the significance level set at p ≤ 0.05.
All applicable institutional, national and international guidelines for the care and use of animals were followed.
During this study, 820 specimens of Trachurus trachurus were examined for Digenea, of which 227 were found to be parasitized, resulting in an overall prevalence of 27.68 %. A total of 1,000 Digenea parasites were collected, belonging to five families: Hemiuridae Looss, 1899; Lepocreadiidae Odhner, 1905; Fellodistomidae Nicoll, 1909; Monorchiidae Odhner, 1911; and Opecoelidae Ozaki, 1925. A single species represented each family: Ectenurus lepidus Looss, 1907 (Fig. 2A), Prodistomum polonii (Molin, 1859) Bray & Gibson, 1990 (Fig. 2 B), Monascus filiformis (Rudolphi, 1819) Looss, 1907 (Fig. 2C), Ancylocoelium typicum Nicoll, 1912 (Fig. 2D), and Pseudopecoeloides chloroscombri (Fischthal & Thomas, 1970) Bartoli, Bray & Gibson, 2003 (Fig. 2E), respectively. The parasitological parameters for each Digenea species are presented in Table 1. Monascus filiformis exhibited the highest prevalence (19.63 %) and mean abundance (0.66), whereas P. chloroscombri recorded the lowest values, with a prevalence of only 1 % and a mean abundance of 0.02. Meanwhile, A. typicum showed the highest mean intensity (4.16), followed closely by P. polonii (4.08). In contrast, P. chloroscombri and E. lepidus displayed the lowest mean intensity, with 1.55 and 1.29 individuals per parasitized host, respectively.
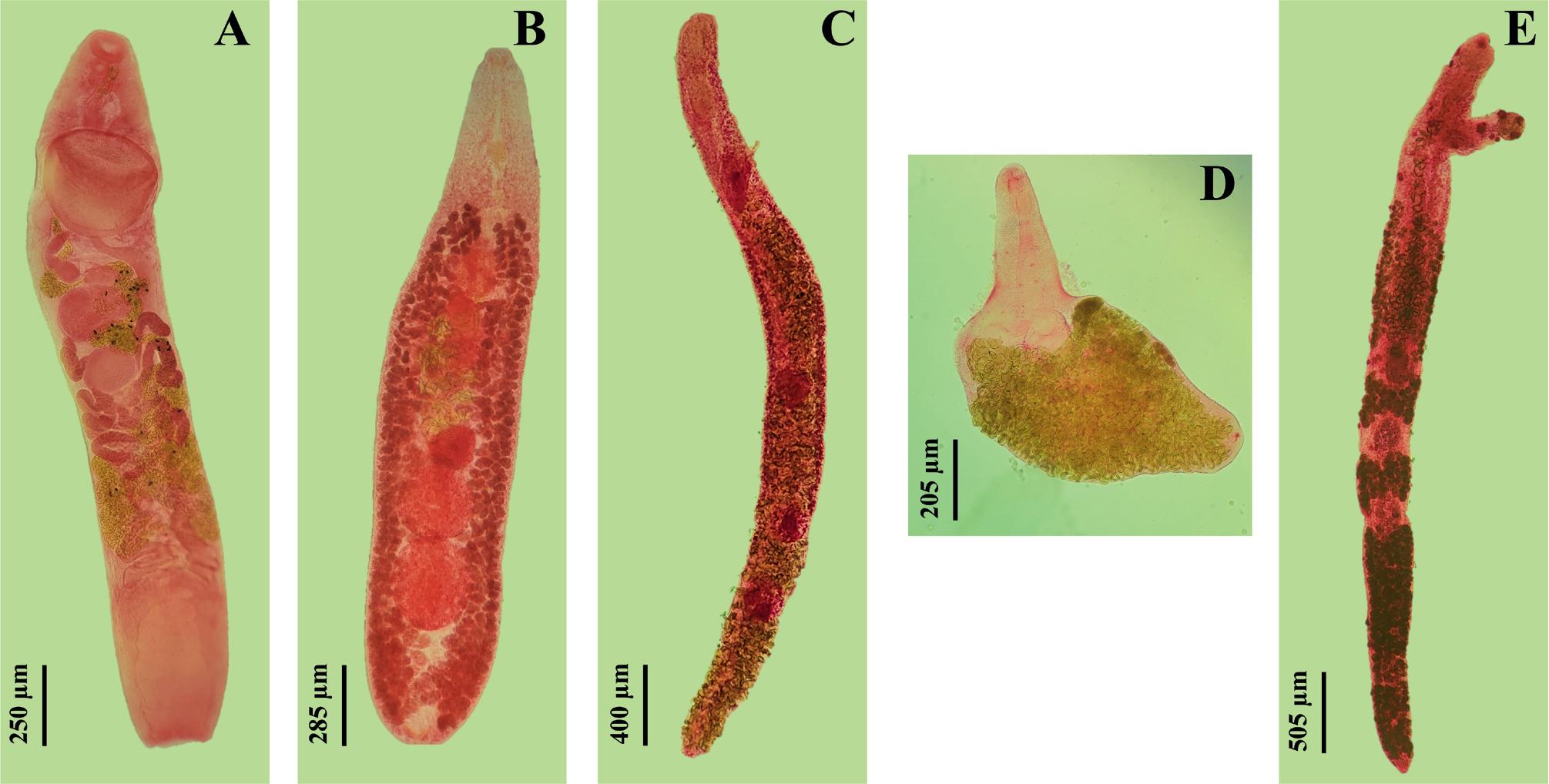
The Digenea infesting Trachurus trachurus from the Algerian coastline Ectenurus lepidus (ventral view) (A); Prodistomum polonii (ventral view) (B); Monascus filiformis (ventral view) (C); Ancylocoelium typicum (ventral view) (D); Pseudopecoeloides chloroscombri (ventral view) (E).
Parasitological parameters of the Digenea species from Trachurus trachurus.
| Host | Parasites species | Number of hosts examined | Number of hosts infected | Number of parasites | Prevalence (P%) | Mean abundance (MA) | Intensity (MI) |
|---|---|---|---|---|---|---|---|
| Trachurus trachurus | Ectenurus lepidus | 820 | 58 | 75 | 7.07 | 0.09 | 1.29 |
| Prodistomum polonii | 39 | 159 | 4.76 | 0.19 | 4.08 | ||
| Monascus filiformis | 161 | 541 | 19.63 | 0.66 | 3.36 | ||
| Ancylocoelium typicum | 50 | 208 | 6 | 0.25 | 4.16 | ||
| Pseudopecoeloides chloroscombri | 11 | 17 | 1 | 0.02 | 1.55 |
It should be noted that not a single fish was infested with all the Digenea species simultaneously. However, every infected fish carried at least one (Table 2): 71.37 % hosted a single species, 19.38 % had two species, 6.61 % had three, and 2.64 % had four. The majority of hosts (71.37 %) harbored a single parasite species, with M. filiformis being the most prevalent (44.05 %), followed by E. lepidus (11.89 %). Co-infestations involving two species occurred in 19.38 % of cases, with common pairings such as M. filiformis and E. lepidus (5.73 %) and M. filiformis and A. typicum (6.17 %). Mixed infestations involving three species were less frequent (6.61 %), with the combination of M. filiformis, E. lepidus, and P. polonii (3.52 %) being the most observed. The rarest cases were four-species infestations (2.64 %). Overall, M. filiformis was the dominant species across both single-species and multi-species infestations.
Parasitic associations in Trachurus trachurus.
| Species composition | Number of infected hosts | P% | |
|---|---|---|---|
| n | P % | ||
| M. filiformis | 100 | 44.05 | 71.37 |
| E. lepidus | 27 | 11.89 | |
| P. polonii | 7 | 3.08 | |
| A. typicum | 20 | 8.81 | |
| P. chloroscombri | 8 | 3.52 | |
| M. filiformis - E. lepidus | 13 | 5.73 | 19.38 |
| M. filiformis - P polonii | 12 | 5.29 | |
| M. filiformis - A. typicum | 14 | 6.17 | |
| M. filiformis - P chloroscombri | 1 | 0.44 | |
| E. lepidus - A. typicum | 1 | 0.44 | |
| P. polonii - A. typicum | 3 | 1.32 | |
| M. filiformis - E. lepidus - P. polonii | 8 | 3.52 | 6.61 |
| M. filiformis - E. lepidus - A. typicum | 3 | 1.32 | |
| M. filiformis - P. polonii - A. typicum | 4 | 1.76 | |
| M. filiformis - E. lepidus - P. polonii - A. typicum | 4 | 1.76 | 2.64 |
| M. filiformis - E. lepidus - P. polonii - P. chloroscombri | 1 | 0.44 | |
| M. filiformis - E. lepidus - A. typicum - P. chloroscombri | 1 | 0.44 | |
The number of T. trachurus specimens examined varied across seasons, with 24 individuals sampled in autumn, 226 in winter, 505 in spring, and 65 in summer (Table 3). Seasonal variations were evident in parasitological parameters, as the highest prevalence, mean abundance, and mean intensity were recorded during spring, while the lowest values for these parameters were observed in winter.
Parasitism of Trachurus trachurus by Digenea across seasons.
| Host | Seasons | Number of hosts examined | Number of hosts infected | Number of parasites | Prevalence (P%) | Mean abundance (MA) | Mean intensity (MI) |
|---|---|---|---|---|---|---|---|
| Trachurus trachurus | Autumn | 24 | 7 | 19 | 29 | 0.79 | 2.71 |
| Winter | 226 | 27 | 54 | 12 | 0.24 | 2.00 | |
| Spring | 505 | 181 | 858 | 36 | 1.70 | 4.74 | |
| Summer | 65 | 12 | 69 | 18 | 1.06 | 5.75 |
The parasitological parameters for each Digenea species across different seasons are summarized in Table 4. The data showed that E. lepidus and M. filiformis were consistently present throughout all seasons. In contrast, P. polonii and P. chloroscombri were recorded exclusively in winter and spring, while A. typicum was observed only in spring and summer. Statistical analyses further supported these seasonal differences in parasite occurrence. The Chi-square test revealed significant variations in the prevalence of most parasite species: M. filiformis, E. lepidus, P. polonii, and A. typicum (p < 0.001). However, P. chloroscombri showed no significant differences in prevalence (X2 = 2.307, p = 0.511) (Table 5). Similarly, the Kruskal-Wallis test confirmed significant seasonal fluctuations in parasite abundance for the same species (p ≤ 0.001).
Parasitological parameters of the Digenea found in Trachurus trachurus by season.
| Seasons | Autumn | Winter | Spring | Summer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasitological parameters | P% | MA | MI | P% | MA | MI | P% | MA | MI | P% | MA | MI | |
| Parasites species | Ectenurus lepidus | 25 | 0.38 | 1.50 | 2 | 0.03 | 1.20 | 8 | 0.11 | 1.31 | 8 | 0.08 | 1.00 |
| Prodistomum polonii | 0 | 0.00 | 0.00 | 1 | 0.01 | 1.00 | 7 | 0.31 | 4.24 | 0 | 0.00 | 0.00 | |
| Monascus filiformis | 21 | 0.42 | 2.00 | 8 | 0.18 | 2.16 | 26 | 0.85 | 3.32 | 11 | 0.95 | 8.86 | |
| Ancylocoelium typicum | 0 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 10 | 0.41 | 4.29 | 3 | 0.03 | 1.00 | |
| Pseudopecoeloides chloroscombri | 0 | 0.00 | 0.00 | 1 | 0.02 | 2.50 | 2 | 0.02 | 1.33 | 0 | 0.00 | 0.00 | |
Analysis of seasonal variations in prevalence and abundance of digenea parasites in Trachurus trachurus using Chi-Square and Kruskal-Wallis tests.
| X2 | Kruskal-Wallis | |||
|---|---|---|---|---|
| Prevalence | Abundance | |||
| X | P | H | P | |
| Ectenurus lepidus | 21.085 | < 0.001* | 21.348 | < 0.001* |
| Prodistomum polonii | 19.288 | < 0.001* | 19.41 | < 0.001* |
| Monascus filiformis | 32.638 | < 0.001* | 32.607 | < 0.001* |
| Ancylocoelium typicum | 27.51 | < 0.001* | 27.588 | < 0.001* |
| Pseudopecoeloides chloroscombri | 2.307 | 0.511 | 2.293 | 0.514 |
p, significance level;
significant values p ≤ 0.05
The number of T. trachurus specimens examined varied across regions, with the highest sampling effort in the central region (726 individuals), followed by the east (68 individuals) and the west (26 individuals) (Table 6). This regional variation was reflected in the parasitological parameters, as the central region exhibited the highest prevalence, mean abundance, and mean intensity. In contrast, the lowest values were recorded in the west.
Parasitism of Trachurus trachurus by Digenea across regions.
| Host | Regions | Number of hosts examined | Number of hosts infected | Number of parasites | Prevalence (P%) | Mean abundance (MA) | Mean intensity (MI) |
|---|---|---|---|---|---|---|---|
| Trachurus trachurus | Center | 726 | 209 | 953 | 29 | 1.31 | 4.56 |
| East | 68 | 13 | 41 | 19 | 0.60 | 3.15 | |
| West | 26 | 5 | 6 | 19 | 0.23 | 1.20 |
A detailed overview of the parasitological parameters for each Digenea species across regions is presented in Table 7. While E. lepidus and P. polonii were found in all three regions, M. filiformis, A. typicum, and P. chloroscombri were restricted to the central and eastern regions. Statistical analysis confirmed significant regional differences in parasite prevalence and abundance. The Chi-square test indicated that M. filiformis (p < 0.001) and A. typicum (p = 0.018) exhibited notable variations across regions, whereas the prevalence of the other species remained consistent (p > 0.05). Likewise, the Kruskal-Wallis test revealed significant differences in abundance for M. filiformis (H = 17.922, p < 0.001) and A. typicum (H = 7.465, p = 0.024). However, the abundance of E. lepidus, P. polonii and P. chloroscombri remained stable between regions (p > 0.05) (Table 8).
Parasitological parameters of the Digenea found in Trachurus trachurus by regions.
| Areas | Center | East | West | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parasitological parameters | P% | MA | MI | P% | MA | MI | P% | MA | MI | |
| Parasites species | Ectenurus lepidus | 7 | 0.09 | 1.31 | 3 | 0.03 | 1.00 | 15 | 0.19 | 1.25 |
| Prodistomum polonii | 5 | 0.21 | 4.19 | 3 | 0.10 | 3.50 | 4 | 0.04 | 1.00 | |
| Monascus filiformis | 22 | 0.72 | 3.30 | 4 | 0.29 | 6.67 | 0 | 0.00 | 0.00 | |
| Ancylocoelium typicum | 6 | 0.27 | 4.80 | 13 | 0.16 | 1.22 | 0 | 0.00 | 0.00 | |
| Pseudopecoeloides chloroscombri | 1 | 0.02 | 1.60 | 1 | 0.01 | 1.00 | 0 | 0.00 | 0.00 | |
Analysis of regional variations in prevalence and abundance of digenea parasites in Trachurus trachurus using Chi-Square and Kruskal-Wallis tests.
| X2 | Kruskal-Wallis | |||
|---|---|---|---|---|
| Prevalence | Abundance | |||
| X | P | H | P | |
| Ectenurus lepidus | 4.508 | 0.105 | 4.545 | 0.103 |
| Prodistomum polonii | 0.608 | 0.738 | 0.585 | 0.747 |
| Monascus filiformis | 18.423 | < 0.001* | 17.922 | < 0.001* |
| Ancylocoelium typicum | 7.996 | 0.018* | 7.465 | 0.024* |
| Pseudopecoeloides chloroscombri | 0.369 | 0.831 | 0.368 | 0.832 |
p, significance level;
significant values p ≤ 0.05
Applying Sturges’ rule, the fish were classified into ten categories based on both weight and total length. The Spearman correlation test revealed significant negative associations between host weight and both the mean abundance and mean intensity of several parasite species, including E. lepidus, P. polonii, and P. chloroscombri (Table 9). Additionally, a significant negative correlation was observed between host total length and the mean intensity of P. polonii.
The relationship between parasite load and Trachurus trachurus weight and size.
| Mean Abundance | Mean Intensity | |||||||
|---|---|---|---|---|---|---|---|---|
| Weight (g) | Total length (cm) | Weight (g) | Total length (cm) | |||||
| rs | p | rs | p | rs | p | rs | p | |
| Monascus filiformis | 0.433 | 0.211 | 0.28 | 0.434 | -0.306 | 0.306 | -0.018 | 0.96 |
| Ectennrus lepidus | -0.759 | 0.011* | 0.049 | 0.894 | -0.895 | < 0.001** | -0.502 | 0.139 |
| Prodistomum polonii | -0.792 | 0.006** | -0.234 | 0.515 | -0.802 | 0.005** | -0.677 | 0.032* |
| Ancylocoelium typicum | -0.119 | 0.744 | 0.263 | 0.464 | -0.615 | 0.059 | 0.138 | 0.705 |
| Pseudopecoeloides chloroscombri | -0.768 | 0.009** | -0.314 | 0.377 | -0.787 | 0.007** | -0.444 | 0.199 |
p, significance level;
significant values p ≤ 0.05
The current investigation identified five species of Digenea in T. trachurus. As previously mentioned, other studies have been conducted to assess the diversity of these parasites in this host from the Algerian coast. The first was by Amine (1999), who recorded only three species: E. lepidus, M. filiformis, and L. excisum. A later study by Abid-Kachour (2014) also reported three species, two of which were consistent with Amine’s findings. The third species, identified initially as Lecithochirium fusiforme Lühe, 1901, was newly recorded. However, according to the World Register of Marine Species (WoRMS), this name is not valid, and the correct designation is Lecithochirium grandiporum (Rudolphi, 1819) Lühe, 1901. More recently, Ichalal et al. (2017) identified eight species, including one unidentified species and four additional Digenea. In the present study, we recorded five species, among which A. typicum represents a novel addition to the Digenean fauna of this host in Algeria. Consequently, our findings contribute to expanding the known biodiversity of Digenean parasites infesting T. trachurus in Algerian waters, increasing the number of documented species from nine to ten. Nevertheless, these parasite species have been widely reported in T. trachurus from various regions (Bartoli et al., 2003; MacKenzie et al., 2004; Bartoli et al., 2005; MacKenzie et al., 2008; Derbel et al., 2012; Feki et al., 2016; Öztürk & Özer, 2016).
The most prevalent and abundant species in this work was M. filiformis, whereas A. typicum and P. polonii exhibited the highest mean intensity values. A comparison with previous research indicates that the prevalence and mean abundance of M. filiformis are consistent with the findings of Ichalal et al. (2017) in Algeria. However, discrepancies were observed when compared to studies conducted by Derbel et al. (2012) in Tunisia, Feki et al. (2016) in the same region, Bartoli et al. (2005) in Corsica, and Öztürk and Özer (2016) along the Turkish Black Sea coast.
The analysis of parasitic associations in T. trachurus revealed that single-species infestations were the most common, a pattern also reported in another work (Boukadoum et al., 2024). Additionally, 17 possible associations among the five parasite species were identified within infected fish. This distribution may result from parasite adaptation to the host’s immune system or interspecific interactions, such as competition (Rigaud et al., 2010; Hoffmann et al., 2016). The latter is particularly relevant when parasites share the same microhabitat and rely on common resources (Telfer et al., 2010), as observed in this study, where all species colonized the host’s digestive tract.
The data collected across different seasons, supported by the Chi-square and Kruskal-Wallis test results, suggest that seasonality significantly influences the prevalence and abundance of most parasite species. This variation may be linked to the life cycle of Digenea, as certain stages, such as miracidia and cercariae, are directly exposed to environmental fluctuations that can impact their development, mobility, and transmission to the next host (Pietrock & Marcogliese, 2003; Parietti et al., 2021). Another factor to consider is the sampling effort for T. trachurus. Although the species was present throughout all seasons, the majority of specimens were collected in spring (505 individuals), compared to lower numbers in autumn (24), winter (226), and summer (65). This disparity could be attributed to the migratory behavior of T. trachurus, as it tends to move closer to the coast during summer and farther offshore (up to 400 m depth) in winter (Abaunza et al., 2003; Rumolo et al., 2017). When analyzing the data across regions along with the statistical results, the observed differences in parasite prevalence and abundance may be partly attributed to sampling effort. Nearly 88 % of the fish were collected from the central region, which could have influenced the regional variation in parasitological parameters. This does not preclude the possible role of environmental factors associated with each region. The eastward-flowing coastal currents from Oran to Algiers (Hemida, 2005) may facilitate the transport of parasite larvae and infectious stages, increasing exposure and transmission potential along the central coastline. Additionally, the central region is characterized by upwelling systems that enhance nutrient availability and promote phytoplankton growth, leading to high chlorophyll a concentration. These conditions support more diverse and abundant trophic networks, which in turn sustain intermediate host populations essential for the completion of the Digenean life cycle (Etsouri et al., 2023). In contrast, the western coastal waters are generally more oligotrophic, with lower productivity and fewer intermediate hosts, potentially limiting parasite development and transmission. Moreover, the central coast is more industrialized and frequently affected by pollution, which may influence parasite–host dynamics (Bendjeddou, 2013). Pollution can suppress the immune response of fish, increasing their vulnerability to parasitic infection (Elie & Girard, 2009), or, conversely, reduce parasite survival, depending on the nature and level of contaminants (Elie & Girard, 2009). Taken together, these ecological and anthropogenic factors likely contribute to the observed regional disparities in Digenean infection levels, beyond the influence of sampling bias alone.
The Spearman correlation test assessing the relationship between host weight and size with parasite mean abundance and mean intensity in T. trachurus revealed a negative correlation, indicating that larger fish tend to have lower parasite loads. This trend may be explained by the host’s diet and the trophic transmission pathways of Digenea. These parasites follow a complex life cycle involving Mollusks as the first intermediate host, fish or invertebrates as the second host, and predatory fish as the definitive host. Infestation occurs when the definitive host consumes an infested second host (Bartoli & Boudouresque, 2007). Studies by Bayhan and Sever (2009) and Nasri et al. (2024) suggest that smaller fish of T. trachurus feed more frequently and on smaller prey like crustaceans (Koç & Erdoğan, 2019), which are often intermediate hosts of digeneans. Additionally, as fish grow, they may develop stronger immune defenses, reducing their exposure to parasites and leading to the observed negative correlation. This perspective is also supported by several authors who have documented similar findings (Kotb et al., 2014; Villalba-Vasquez et al., 2018; Boukadoum & Tazerouti, 2024).
This study provides new insights into the diversity and ecology of Digenea infestations in T. trachurus in Algeria. Based on statistical analyses and parasitological parameters, our findings suggest that environmental factors, host size, and feeding behavior significantly influence parasite distribution. Moreover, this study expands the known diversity of digenean species in T. trachurus, contributing to a broader understanding of parasitic Platyhelminthes in Algerian marine ecosystems. Further investigations are warranted to assess the ecological implications of these patterns and their potential impact on host population dynamics.