Fig 1.
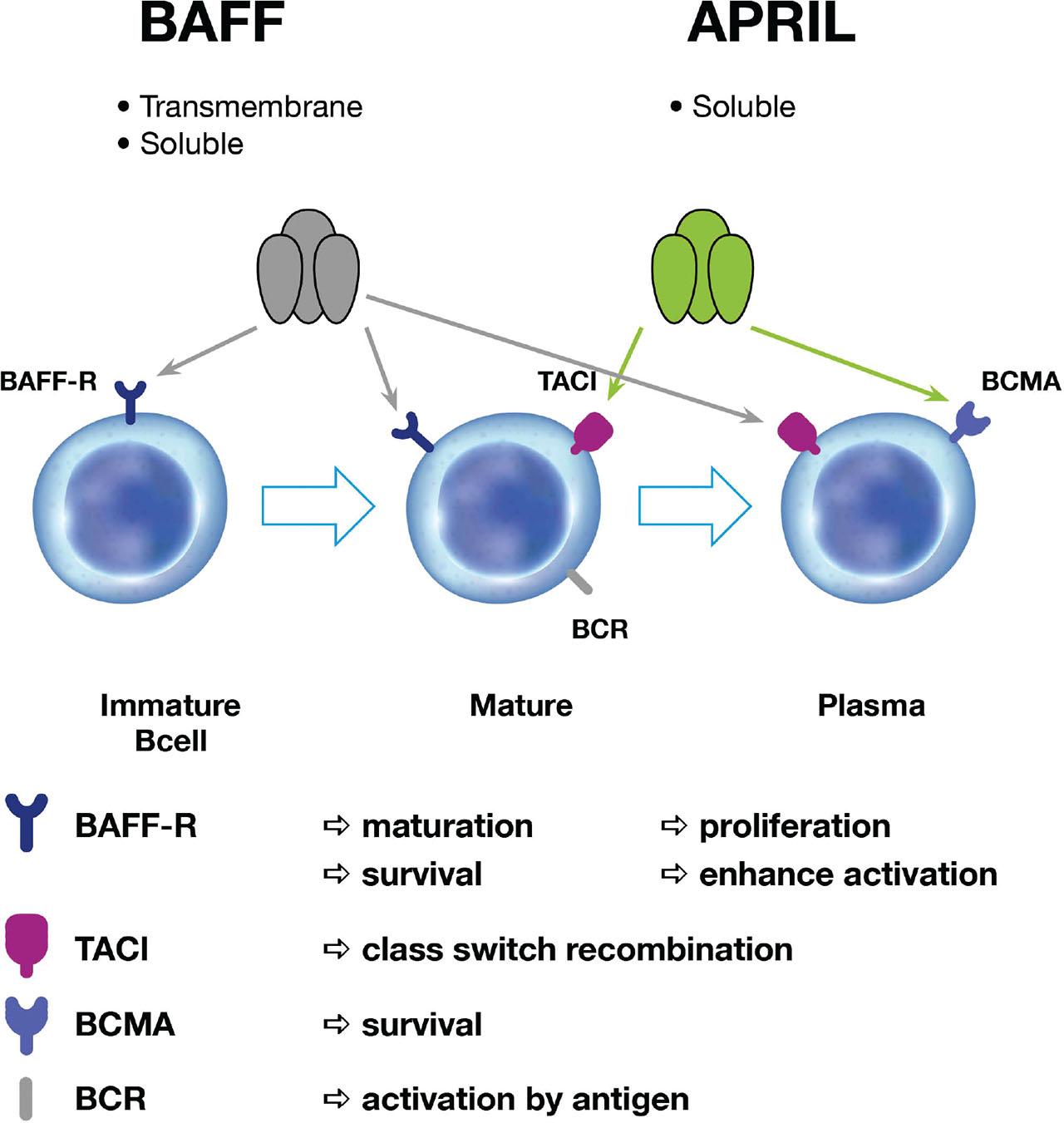
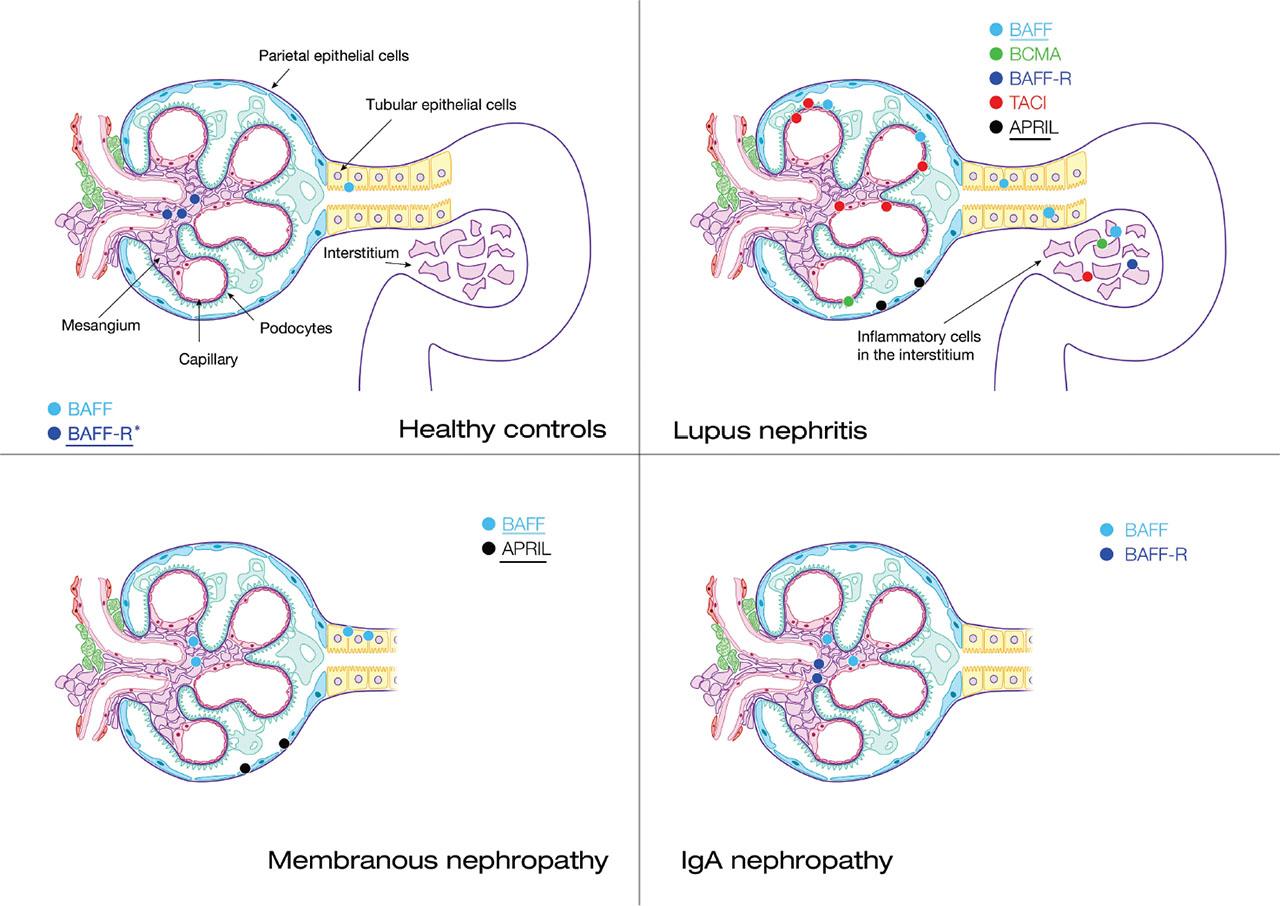
Fig 2.
Biological agents targeting BAFF and APRIL
| Name | Target | Mode of action | Potential application | Research phase | References |
|---|---|---|---|---|---|
| Belimumab | Soluble BAFF | Monoclonal antibody | Approved in SLE, trials in PLA2R + MN | Phase 2 NCT03949855 | Huang et al. (2022) |
| Blisibimod | Soluble BAFF | Peptibody | SLE | No ongoing trials | Chan et al. (2023) |
| Tabalumab | Soluble and membrane-bound BAFF | Human monoclonal antibody | SLE | No ongoing trials | Chan et al. (2023) |
| Ianalumab | BAFF-R on B cells | Monoclonal antibody | SS, SLE |
| Bowman et al. (2022) |
| Atacicept | BAFF and APRIL | Fusion protein (TACI receptor + Ig fragment) | SLE, IgAN |
| Lafayette et al. (2024) |
| Telitacicept | BAFF and APRIL | Fusion protein TACI-Fc | IgAN, MN |
| Cai et al. (2023) and Zhang et al. (2023) |
| Sibeprenlimab | APRIL | Humanized IgG2 monoclonal antibody | IgAN |
| Mathur et al. (2024) |
| BION-1301 (Zigakibart) | APRIL | Monoclonal antibody | IgAN |
| Barratt et al. (2023) |
Recruiting trials involving BAFF-CAR-T cells in autoimmune conditions
| Trial and CAR-T type | Condition | Location | Trial number |
|---|---|---|---|
| Phase I/II open-label, interventional single-arm trial of MB-CART19.1 | Refractory SLE | Germany | NCT06189157 |
| Phase I/II, interventional, 4SCAR-T | Autoimmune diseases | China | NCT05459870 |
| Early Phase I, interventional, CD19-BAFF CAR-T | SLE, systemic sclerosis, dermatomyositis, immune nephritis, neuromyelitis optica | China | NCT06279923 |