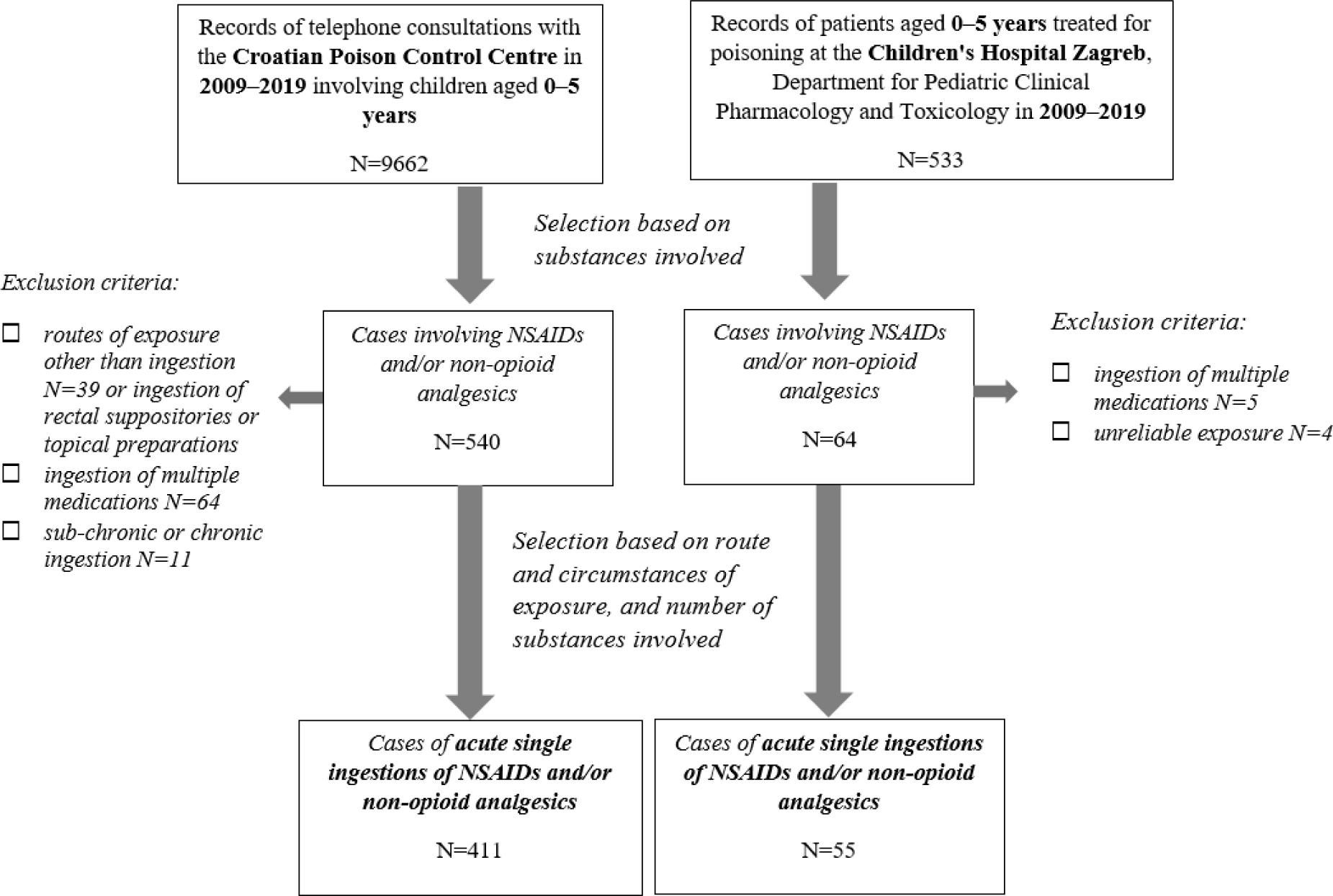
Figure 1
Profile of patients ingesting ibuprofen and paracetamol above maximum daily doses
| Ibuprofen (MDD 30 mg/kg) | Paracetamol (MDD 960 mg) | |||
|---|---|---|---|---|
| CPCC cases (N=121) | CHZ cases (N=15) | CPCC cases (N=35) | CHZ cases (N=4) | |
| Ingested dose in mg/kg, median (range) | 60 (32–270)* | 86 (34–256)* | 86 (36–218)* | 145 (86–203)* |
| Above toxic threshold of 200 mg/kg, N (%) | 2 (2) | 1 (7) | 1 (3) | 1 (25) |
| Symptomatic, N (%) | 7 (6)* | 7 (47)* | 1 (3) | 3 (75) |
| Doses in symptomatic patients, median (range) | not investigated | 72 (34–256) | not investigated | 145 (135–155) |
| Clinical effects, N (%) | ||||
| vomiting | not investigated | 3 (20) | not investigated | 2 (50) |
| diarrhoea | – | 1 (25) | ||
| abnormal laboratory findings | 5 (33)** | 2 (50)*** | ||
Patient demographics and circumstances of exposure (2009–2019)
| CPCC cases (N=411) | CHZ cases (N=55) | |
|---|---|---|
| Age, median (IQR)* | 2.5 (2.0–3.0) | 2.9 (2.2–3.7) |
| Male sex, N (%*) | 188(46) | 32 (53) |
| Drug ingested, N (%) | ||
| ibuprofen | 191(46) | 26 (47) |
| paracetamol | 79 (19) | 14 (25) |
| ketoprofen | 59(14) | 9 (16) |
| diclofenac | 44 (11) | 5 (9) |
| acetylsalicylic acid | 22 (5) | 1 (2) |
| other** | 16 (4) | – |
| Circumstances of exposure | ||
| unsupervised child ingested the drug left within reach | 386(94) | 54 (98) |
| dosing error made by parents/caregivers | 25 (6) | 1 (2) |