Figure 1
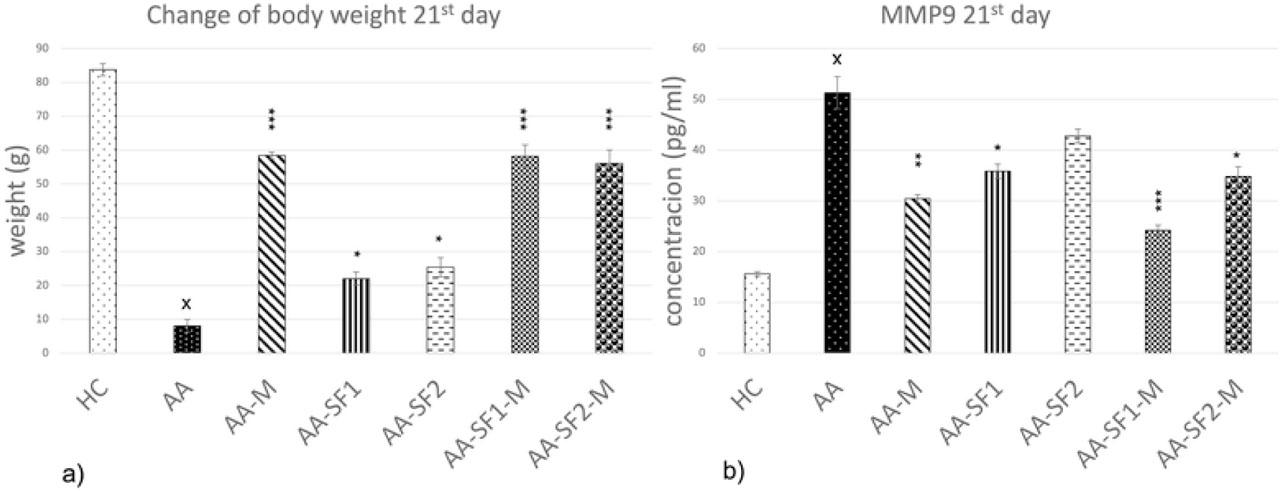
Figure 2
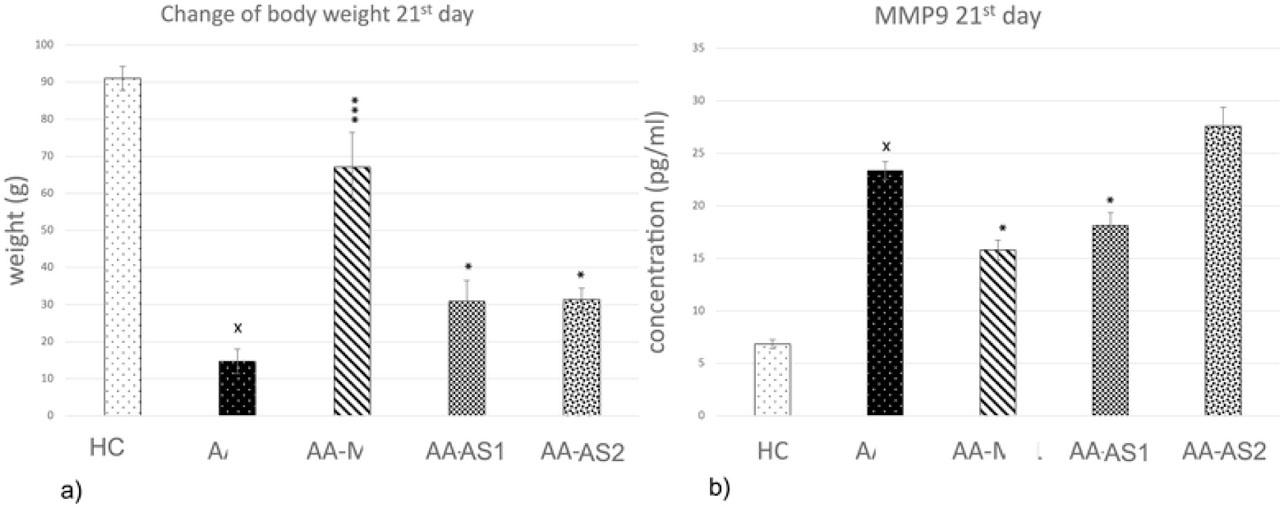
Figure 3
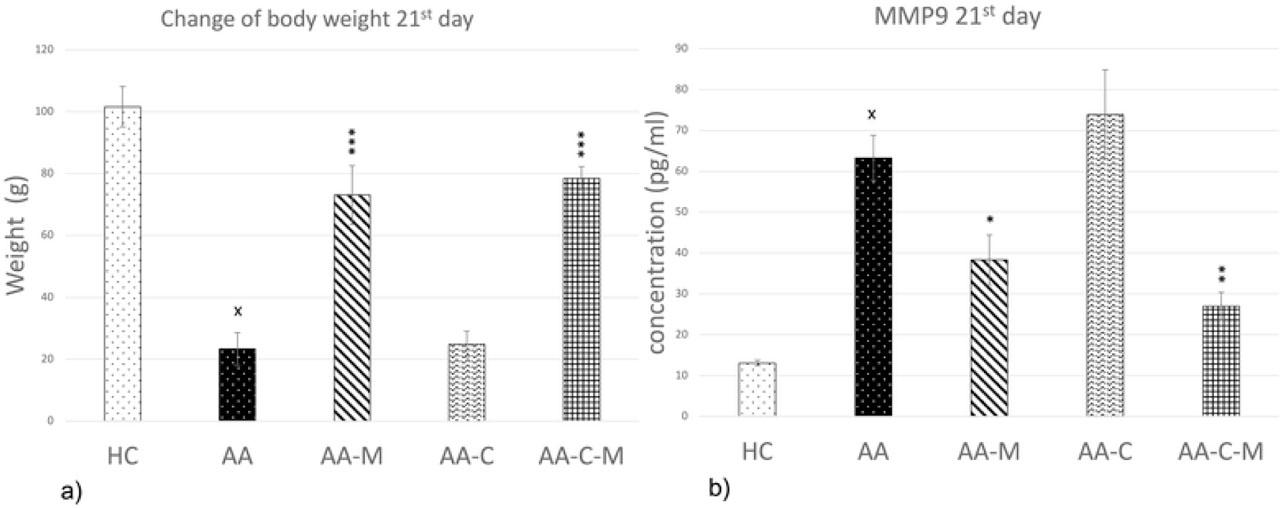
Summary of experimental design of three different experiments (a, b, and c)_
| Group | Treatment | Peroral dose |
|---|---|---|
| Group 1 a, b, c: healthy controls (HC) | Vehiculum | 0.5 ml daily |
| Group 2 a, b, c: adjuvant arthritis (AA) untreated | Vehiculum | 0.5 ml daily |
| Group 3 a, b, c: AA + treatment | Methotrexate (M) | 0.3 mg/kg twice a week |
| Group 4 a: AA + treatment | Saffron extract (SF1) | 25 mg/kg daily |
| Group 5 a: AA + treatment | Saffron extract (SF2) | 50 mg/kg daily |
| Group 6 a: AA + treatment | SF1+ M | 25 mg/kg + 0.3 mg/kg |
| Group 7 a: AA + treatment | SF2 + M | 50 mg/kg + 0.3 mg/kg |
| Group 4 b: AA + treatment | Astaxanthin (AS1) | 1 mg/kg daily |
| Group 5 b: AA + treatment | Astaxanthin (AS2) | 5 mg/kg daily |
| Group 4 c: AA + treatment | Carnosic acid (C) | 100 mg/kg daily |
| Group 5 c: AA + treatment | C + M | 100 mg/kg + 0.3 mg/kg |