Figure 1.
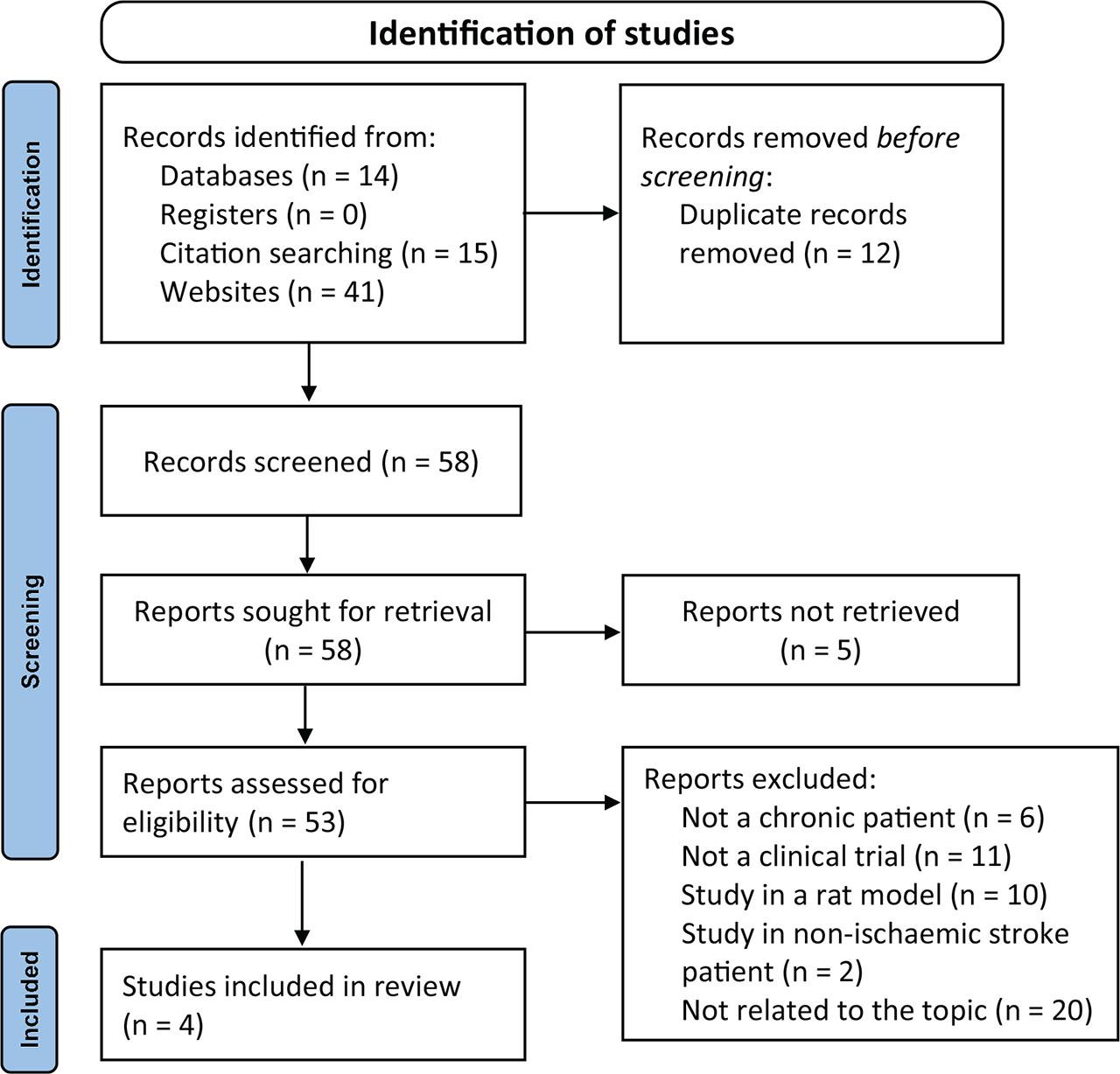
Figure 2.
![MSCs can be sourced from bone marrow, adipose tissue, umbilical cord, and dental pulp. These cells can treat ischemic stroke by secreting neuroprotective factors, acting as immunomodulators, and promoting angiogenesis and neurogenesis [32,33,34]. Created with BioRender.com. MSCs, mesenchymal stem cells.](https://sciendo-parsed.s3.eu-central-1.amazonaws.com/6722aa805313275c675673a1/j_abm-2024-0027_fig_002.jpg?X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Credential=ASIA6AP2G7AKHNKHNVAF%2F20251210%2Feu-central-1%2Fs3%2Faws4_request&X-Amz-Date=20251210T102958Z&X-Amz-Expires=3600&X-Amz-Security-Token=IQoJb3JpZ2luX2VjEAgaDGV1LWNlbnRyYWwtMSJHMEUCIQCZ35d8Vjl9gVT3R32xCY4xAZpFqa7jRB8ylhCjZvDqdQIgRwygqpMdndDj5T49TIdxhaH467hMNful98sExCY8aowqxgUI0f%2F%2F%2F%2F%2F%2F%2F%2F%2F%2FARACGgw5NjMxMzQyODk5NDAiDPHzvHdj4jOTnhNq9CqaBbXxSwy4DVEkHRAfFCzsmdrgI%2FasjEJQj1TfhCCc0rPZjn2UDdK%2Bkqm3jb2aOINAyQXp6%2BOotr%2Ffp96weqfk65%2FXNyEXv9kkQzXAsCo3zeLSEwjjAUw0L4VN7%2Bo7GD5YNhl%2BF89jTCuSd%2BtL%2FM5O1VNR3KHWF%2Ft8Qp%2FYJsAfaa7%2FdDVb%2FcIobIFGiKdvEJlXp9MDRdx1ChqV1JbSV5gpZlly1EQUZPE84EFyh9IIBV7E8TlerNtHO1t1LasuTTQafHwPjFzXNOgnlQ6IaT7TuSSV4gAYsikFu45O%2FmAV6vQU12Fb21t%2BXqjxaUZx5Z52nmLbu52gprdGM7Aj9h9ueO8n3tVaamtZH9MTRv9nvb1MKTFlUqm62IcyL1MHCMfwx3Th2L90t%2BG5Za2JvtB04H8pQyBlwCQ9hsVn5Ik9PbLke1mYVusc5r49Fxp1Y5EgPmnow4pUcjP5UwVvZqKExLjnn0OmTP2D0Zh1ogPyFXqFNB91vuvENA7cAgkSCJNvHta4Pen8NzQ7OeWpzhYsf2hmZDZgXq8oZzfsByiyy69l7zeYH%2Bvc9hUulIWF6TbeJVbSgnhMzYqDLBUhrSLCSBac2gzF2bLznKNOMnNZj2lEVvl7SW7bAcElZLOD0o40O0M4GHEGw6sgcOB5xiASvcV9cRSzrqrgATjjA5FLgxP29EBSEVBh0TfUjNNs8UzAv6UwZwCID3TlYuByK6unxWtBc7duqt%2BtBe4AV4IxCjuXy0RoxjrXpRgJX3a0VSonAljnko6LXNOtS%2B5faqAC4X9bBXiifVymirZlFmWCm%2FGKjxgGgZKsg6OMBeje0wqSjRTQ4vc1T4GHriPIuIiKnjrnDJrAxMBXr3YZErZCix%2B7amOuYOnqW%2F8E%2BDCR1OTJBjqxAaOZlmtmSqqOFSC1RX5xPxIhi4w7ftD68uJeicnAo7zSo2Bb6%2FRG0uhOwppCr9rRJwusmT2dIxH53C2fYCR3L6msfaJJo2FhEJ%2FbNcJzidUgprk1Gs%2BJ9W0UykzMCKsyzwjom6UYvHbUjcdzbTRd%2BIfgdmkzS0tQDHo5ncsQlZwfALeznKRXTDqLEJXQL%2FzJQKet2ax1lnrMDE8%2BeTlho2xpFQ1cahd696MPlfrFIsqDTg%3D%3D&X-Amz-Signature=d784045da2bdb93c4113f490f1e8a18a7b8281f1af870249bf2f2357cb92b8f9&X-Amz-SignedHeaders=host&x-amz-checksum-mode=ENABLED&x-id=GetObject)
Characteristics of extracted studies
| References | Location | Study design | Sample | Time from stroke to therapy | Cell source | Route of administration | Dosage | Outcome measure | Follow-up time | Comparison | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steinberg et al. [16] | USA | A non-randomized, open-label, single-arm study | 18 | Minimum 6 months | Allogeneic cells | Stereotactic intracranial injection | 2.5 × 106, 5.0 × 106, or 10 × 106 | ESS, NIHSS, FMA, F-M Motor Scale Score, mRS | On month 24 | – | 6 |
| Levy et al. [15] | USA | A non-randomized, open-label, single-arm study | 36 | Minimum 6 months | Allogeneic cells | Intravenous | Phase I = 0.5 × 106, 1.0 × 106, and 1.5 × 106; phase 2 = 1.5 × 106/kg of body weight | NIHSS, BI, MMSE, Geriatric Depression Scale | On day 2, 3, 4, and 10, and on month 1, 3, 6, 9, and 12 | – | 6 |
| Bhasin et al. [17] | India | A non-randomized, open-label study | MSCs group = 6; control group = 6 | Minimum 3 months | Autologous cells | Intravenous | 50–60 × 106 | FMA, mBI | On week 8, 24, 78, 156, and 208 | Placebo | 7 |
| Chiu et al. [14] | Taiwan | A non-randomized, open-label, single-arm study | 3 | Minimum 6 months | Autologous cells | Stereotactic intracranial injection | 1.0 × 108 | NIHSS, mFMA sensation, BI, Berg Balance Scale | On month 6 | – | 6 |
Search keywords
| Electronic database name | Keywords |
|---|---|
| PubMed | (Chronic[Title/Abstract]) AND ((Cerebral Ischemia[Title/Abstract]) OR (Ischemic Stroke[Title/Abstract]) OR (Middle Cerebral Artery Occlusion[Title/Abstract]) OR (Cerebral Infarction[Title/Abstract]) OR (Ischemic Brain Injury[Title/Abstract])) AND ((Bone Marrow Derived Stem Cells[Title/Abstract]) OR (Adipose Derived Stem Cells[Title/Abstract]) OR (Umbilical-Cord Derived Stem Cells[Title/Abstract]) OR (Mesenchymal Stem Cells[Title/Abstract])) |
| PubMed Central | (Chronic[Title/Abstract]) AND ((Cerebral Ischemia[Title/Abstract]) OR (Ischemic Stroke[Title/Abstract]) OR (Middle Cerebral Artery Occlusion[Title/Abstract]) OR (Cerebral Infarction[Title/Abstract]) OR (Ischemic Brain Injury[Title/Abstract])) AND ((Bone Marrow Derived Stem Cells[Title/Abstract]) OR (Adipose Derived Stem Cells[Title/Abstract]) OR (Umbilical-Cord Derived Stem Cells[Title/Abstract]) OR (Mesenchymal Stem Cells[Title/Abstract])) |
| Google Scholar | allintitle: mesenchymal stem cells chronic stroke |
| CENTRAL | “Intervention (‘Mesenchymal stromal cells’ OR ‘Bone marrow derived mesenchymal stromal cells’ OR ‘Umbilical cord mesenchymal stem cells’ OR ‘Human dental pulp mesenchymal stem cells’) AND Population (‘Ischemic Stroke’ OR ‘Occlusion Of Artery’ OR ‘Ischemic Stroke’ OR ‘Cerebral Infarction’)” |
| ClinicalTrials.gov | Condition “Chronic Ischemic Stroke” AND Intervention “Mesenchymal stem Cells” |
Summary of efficacy and safety outcomes
| References | Efficacy outcome | Safety outcome | Study limitation |
|---|---|---|---|
| Steinberg et al. [16] | The mean ± SD of baseline NIHSS total score, 9.3 ± 1.7, significantly improved at 12 months (−1.9, 95% CI: −2.6 to −1.1, P < 0.001) and 24 months (−2.1, 95% CI: −3.3 to −1.0, P < 0.01). Similarly, the mean ± SD of baseline FM total score, 133.6 ± 20.9, showed significant improvement at 12 months (19.2, 95% CI: 11.4–27.0, P < 0.001) and 24 months (19.4, 95% CI: 9.9–29.0, P < 0.01). | At 24 months, all patients in the cohort experienced at least one AE. Common AEs and their respective percentages included headache related to surgical procedures (88.9%), nausea (33.3%), depression (22.2%), muscle spasticity (22.2%), vomiting (22.2%), increased blood glucose (16.7%), elevated C-reactive protein levels (16.7%), constipation (16.7%), fatigue (16.7%), pain in extremity (16.7%), and urinary tract infection (16.7%). Most of these AEs were mild (11.1%) or moderate (50.0%) in intensity. | Small sample size and the used of uncontrolled study design. |
| Levy et al. [15] | The median (interquartile range) of baseline NIHSS total score, 8 (6.5–10), significantly improved at 6 months (−1, P < 0.0001) and 12 months (−2, P < 0.001). The mean ± SD of baseline BI score, 65 ± 28.7, showed a notable gain of 6.8 points at 6 months (P = 0.0002) and further escalated to 10.8 points at 12 months (P < 0.001). The percentage of patients achieving an excellent functional outcome (BI score ≥95) increased from 11.4% at baseline to 27.3% at 6 months and 35.5% at 12 months. | Out of 15 serious AEs, all were deemed unrelated or unlikely related to the investigational product. Among the 109 reported AEs, two were possibly related: a mild urinary tract infection and mild intravenous site irritation, both resulting in full recovery. Additional details are available in the study report. | There was no control group and no study regarding the mechanism of action. |
| Bhasin et al. [17] | In the MSCs group, both FM and mBI scores increased from baseline to the 208th week. The control group also showed notable improvement from baseline to the 4-year assessment. Baseline characteristics were aligned (P > 0.05), confirming comparability for evaluating therapy effectiveness at 8, 24, 78, 156, and 208 weeks. Notably, only mBI exhibited statistical significance at 208 weeks (95% CI: −12.9 to 0.49, P = 0.05) and at 156 weeks (95% CI: −1.26 to 1.76, P = 0.04). No significant difference in FM scores was observed at the 4-year examination (95% CI: −3.01 to 2.01, P = 0.19). | After 9 months post-transplantation, one patient reported a skin allergy/rash. Subsequently, the patient was hospitalized for the issue, but it was determined that the infection was not related to the administered cells. | Small sample size. |
| Chiu et al. [14] | At 6 months, all patients exhibited improved motor functions (2–5 points) and sensory functions (0–2 points) compared to the initial NIHSS score. FMA scores indicated enhancements in both motor and sensory functions, while BI results highlighted improvements in daily life activities. | AEs were reported, yet the authors concluded that they were unrelated to the administration of cell therapy. Unfortunately, specific details about these AEs were not provided. | The sample size is limited and there is no group for comparison. |