Figure 1.
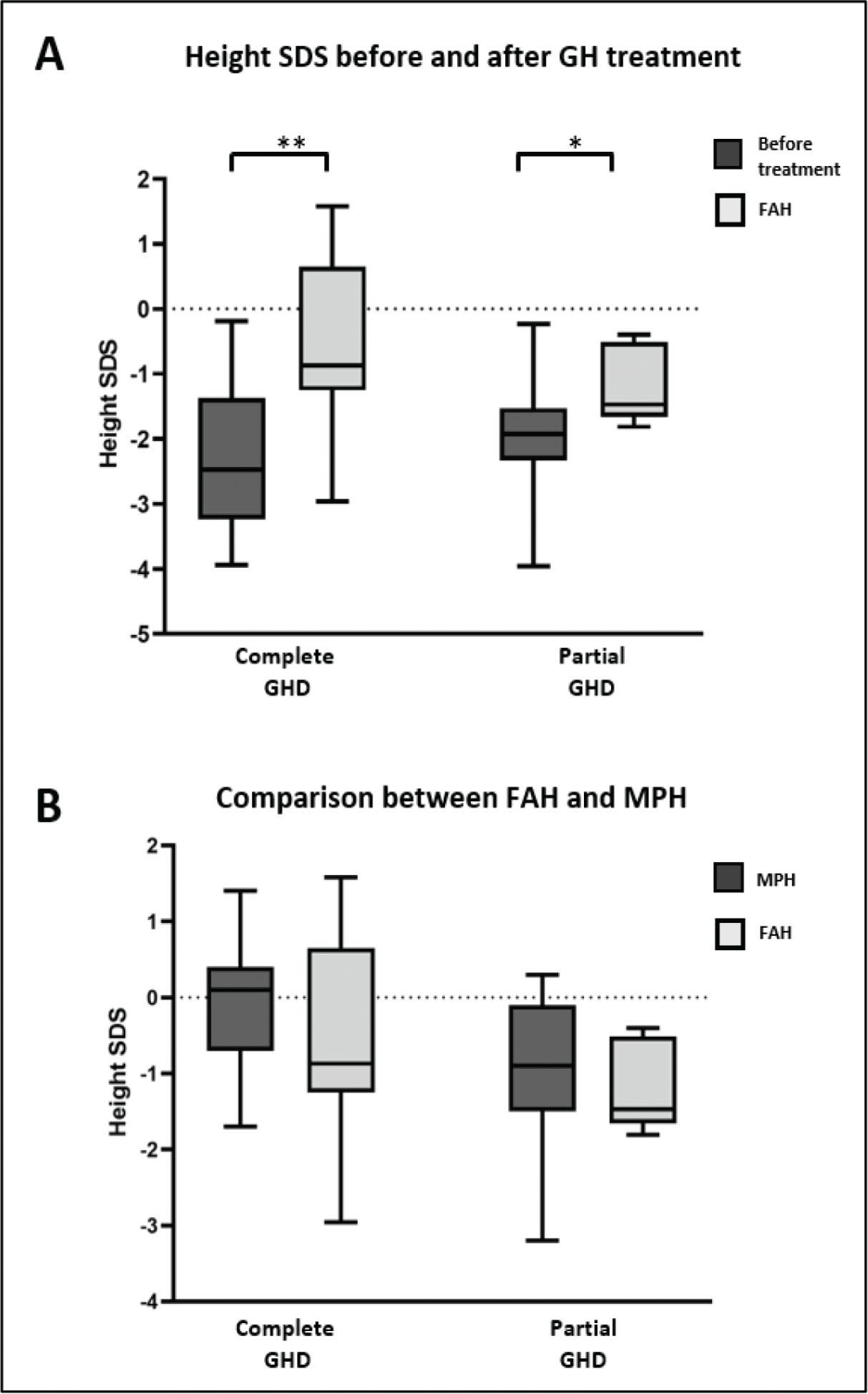
Clinical characteristics before and after rhGH treatment categorized according to the GH status as complete vs_ partial GHD
| Overall (n = 40) | Complete GHD (n = 20) | Partial GHD (n = 20) | P | |
|---|---|---|---|---|
| Sex, n (%) | 1.0 | |||
| Male | 25 (62.5) | 12 (60.0) | 13 (65.0) | |
| Female | 15 (37.5) | 8 (40.0) | 7 (35.0) | |
| Baseline | ||||
| Age (years) | 8.9 (3.0) | 9.6 (3.4) | 8.2 (2.6) | 0.1 |
| Bone age (years)* | 4.5 [3.0, 7.8] | 7.0 [2.8, 9.0] | 4.3 [3.3, 4.9] | 0.4 |
| Height SDS | −2.13 (0.94) | −2.30 (1.07) | −1.96 (0.78) | 0.3 |
| Weight SDS | −1.19 (1.35) | −1.04 (1.50) | −1.34 (1.21) | 0.5 |
| HV (cm/year)† | 3.55 [3.00, 4.80] | 3.30 [2.95, 4.08] | 4.50 [3.18, 4.93] | 0.1 |
| MPH SD | −0.49 (1.0) | −0.16 (0.087) | −0.83 (1.02) | 0.03 |
| Peak GH (ng/mL) | 4.63 (2.98) | 2.56 (1.80) | 6.70 (2.44) | <0.001* |
| IGF-1 SDS | −0.97 (1.56) | −1.12 (1.93) | −0.83 (1.11) | 0.6 |
| IGFBP-3 SDS | −0.79 (1.44) | −1.08 (1.25) | −0.51 (1.60) | 0.3 |
| MPHD, n (%) | 10 (25.0) | 9 (45.0) | 1 (5.0) | 0.01* |
| TSH deficiency | 7 (17.5) | 6 (30.0) | 1 (5.0) | 0.1 |
| DI | 7 (17.5) | 6 (30.0) | 1 (5.0) | 0.1 |
| ACTH deficiency | 5 (12.5) | 5 (25.0) | 0 (0.0) | 0.1 |
| Hypogonadism | 7 (17.5) | 7 (35.0) | 0 (0.0) | 0.01* |
| MRI finding, n (%) | 0.1 | |||
| Normal | 21 (52.5) | 9 (45.0) | 12 (60.0) | |
| Abnormal | 7 (17.5) | 6 (30.0) | 1 (5.0) | |
| Not performed | 12 (30.0) | 5 (25.0) | 7 (35.0) | |
| Age at puberty (years)† | 12.8 [11.9, 13.8] | 13.1 [12.5, 14.0] | 12.0 [11.2, 13.3] | |
| Male | 13.3 (1.7) | 13.7 (2.2) | 12.9 (0.9) | 0.3 |
| Female | 12.2 (1.5) | 13.2 (0.8) | 10.5 (0.8) | <0.001* |
| rhGH treatment | ||||
| Duration of treatment (years) | 4.9 (3.3) | 5.7 (3.2) | 4.0 (3.3) | 0.1 |
| rhGH dosage (μg/kg/d)† | 28.4 [21.3, 31.7] | 26.6 [19.8, 29.1] | 30.9 [28.0, 31.9] | 0.02* |
| Discontinue rhGH | ||||
| Age (years)† | 15.3 [14.4, 16.0] | 15.3 [14.9, 16.2] | 15.3 [14.0, 16.0] | 0.5 |
| Bone age (years)† | 14.4 [14.0, 16.0] | 14.4 [14.0, 16.0] | 14.5 [14.0, 15.0] | 0.9 |
| FAH (cm) | ||||
| Male | 166.2 (5.9) | 168.0 (6.0) | 161.4 (1.8) | 0.003 |
| Female | 152.5 (6.1) | 152.2 (7.2) | 153.2 (2.8) | 0.4 |
| FAH SDS† | −0.87 [–1.56, −0.14] | −0.65 [–1.22, 0.70] | −1.47 [–1.66, −0.78] | 0.2 |
| Height SDS gain (start-stop of rhGH) | 1.43 (1.00) | 1.62 (1.09) | 0.91 (0.47) | 0.1 |
| FAH-MPH SDS | −0.64 (1.38) | −0.55 (1.7) | −0.87 (1.76) | 0.6 |
Proportion of retesting GH results categorized according to the GH status and hormonal deficiency
| Persistent GHD (n = 11) | Transient GHD (n = 13) | P | |
|---|---|---|---|
| GH status, n (%) | 0.4 | ||
| Complete GHD | 8 (53.3) | 7 (46.7) | |
| Partial GHD | 3 (33.3) | 6 (66.7) | |
| Hormonal defects, n (%) | 0.002* | ||
| Isolated GHD | 3 (20.0) | 12 (80.0) | |
| MPHD | 8 (88.9) | 1 (11.1) |